The CDC and its Advisory Committee on Immunization Practices (ACIP), together with the AAFP and other medical professional organizations, have released the 2017 adult and childhood immunization schedules.
Childhood and Catch-up Schedule Highlights
- Patients ages 11-12 receive two doses of HPV9; however, this dosing schedule can begin as early as age 9 and as late as ages 13-14. Although it is recommended that the second dose of the two-dose schedule be administered six to 12 months after the first dose, the minimum interval between the first and second doses is five months.This recommendation follows the FDA’s approval(www.fda.gov) of a request this past October to add a two-dose schedule of HPV9 for adolescents ages 9-14 as an alternative to the previously licensed three-dose schedule.
- For patients initiating HPV9 vaccination at or after age 15, the recommended immunization schedule is three doses. The second dose should be administered one to two months after the first dose, and the third dose should be administered six months after the first dose.
- The ACIP’s updates for the MenB vaccine apply only to the MenB-FHbp (Trumenba). For patients at increased risk for meningococcal disease and for use during serogroup B outbreaks, three doses of MenB-FHbp should be administered at ages 0, 1-2 months and 6 months. When given to healthy adolescents who are not at increased risk for meningococcal disease, two doses of MenB-FHbp should be administered at 0 and 6 months. If the second dose is given at an interval of less than 6 months, a third dose should be given at least 6 months after the first dose.
- For all medically stable infants weighing greater than or equal to 2,000 grams at birth and born to HBsAg (hepatitis B surface antigen)-negative mothers, the first dose should be administered within 24 hours of birth (instead at previously recommended “at hospital discharge.” Only single-antigen HepB vaccine should be used for the birth dose.
- Influenza vaccine: even though live attenuated influenza vaccine (LAIV; FluMist) was taken off the market this flu season in the United States, the ACIP still hasn’t announced a plan for its use during the 2017-2018 season. The group is, however, scheduled to discuss the vaccine at its Feb. 22-23 meeting(www.cdc.gov).
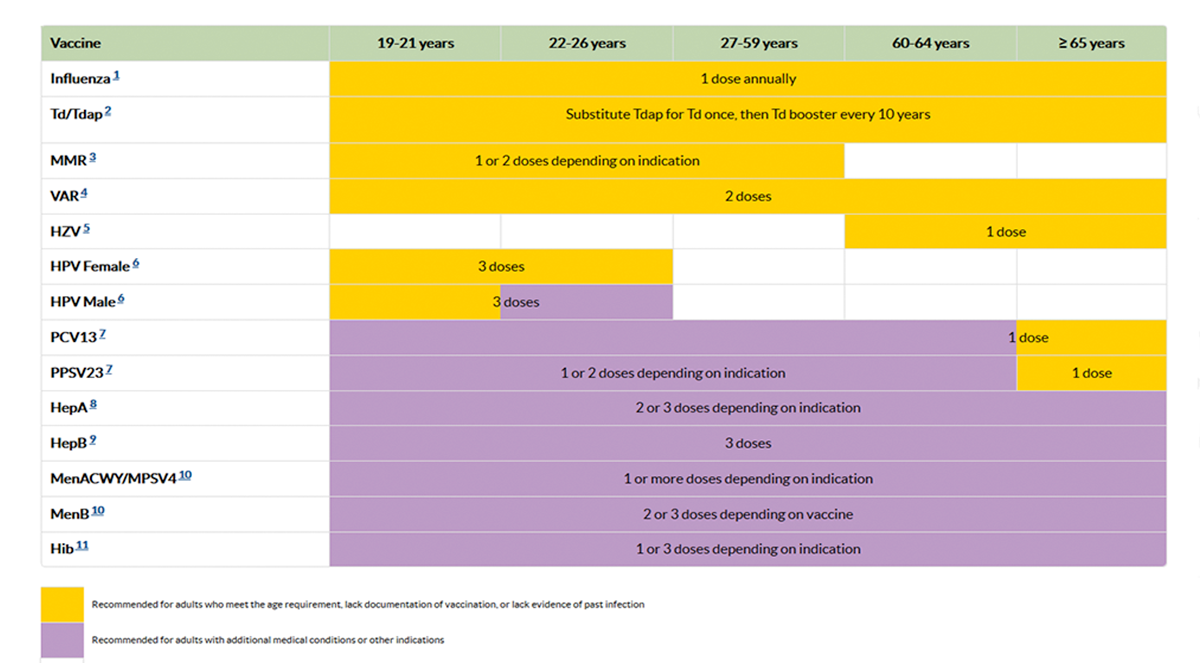
Adult Schedule Highlights
The 2017 adult immunization schedule has been revised to be easier to read, with a new format that includes landscape formatting, cleaner graphics, use of abbreviations and a larger font in the footnotes.
- Patients with a history of egg allergy that manifests with symptoms other than hives — such as those who experience angioedema, respiratory distress, lightheadedness or recurrent emesis, or who require epinephrine or another emergency medical intervention — may receive age-appropriate inactivated (IIV) or recombinant influenza vaccine (RIV).
- Adults with egg allergy who experience only hives after exposure to egg should receive IIV or RIV.
- The ACIP recommends the HepB vaccine for adults with chronic liver disease, including those with hepatitis C infection and liver function enzyme levels twice the upper limit. This recommendation also includes patients with cirrhosis, fatty liver disease, alcoholic liver disease and autoimmune hepatitis.
- HPV recommendation for adults didn’t actually change. Teens and young adults who start the series at ages 15-26 will continue to need three doses of HPV vaccine.
View complete immunization schedules by age group.