Delaying the second dose of the mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) Covid-19 vaccines for 9 to 15 weeks after the first dose may help avoid more hospitalizations, infections, and death than following the recommended schedules, according to results of a modelling study published in PLOS Biology.
However, these improved Covid-19 outcomes were only achieved when the upper bounds of vaccine efficacy were reached; when researchers modeled vaccine efficacy at the lower bounds of vaccine efficacy, delaying second dosing of the vaccines had no advantage compared with the recommended schedules in reducing infections.
These conclusions go against current recommendations from the CDC’s Advisory Committee on Immunization Practices (ACIP), which call for the second vaccination with the mRNA-1273 Covid-19 vaccine at four weeks after first vaccination and a delay of three weeks for second vaccination with the BNT162b2 Covid-19 vaccine.
For the study, Alison P. Galvani, PhD, of the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, and fellow authors developed a model of Covid-19 transmission to compare the effects of two vaccination strategies. They used all available evidence in published clinical trials, FDA briefing documents, and vaccination campaigns.
“[W]e employed an agent-based model of Covid-19 transmission and vaccination to compare the epidemiological impact of tested and [delayed second dose] DSD vaccination schedules, considering a range of preexisting immunity accrued since the emergence of Covid-19. We determined the optimal timing for administering the second dose based on vaccine efficacy estimated in clinical trials and population-level studies following first and second doses,” they explained.
In a model in which the efficacy of the first does did not wane for up to 18 weeks after administration, a delayed second dose strategy and a daily rate of 30 vaccine doses per 10,000 population helped avoid more infections, hospitalizations, and deaths compared with the recommended schedules for both vaccines. The greatest reduction in severe outcomes was achieved with a 12- to 15-week delay in administering the second doses of these vaccines.
But, “[a]s the daily number of vaccine doses increases, the maximum benefits of a DSD strategy in averting hospitalizations and deaths would be achieved with a shorter delay in administering the second dose,” noted Galvani et al.
They found that for the mRNA-1273 Covid-19 vaccine, a delay of at least nine weeks could be optimal for effectiveness and avoid an additional 17.3 infections, 0.69 hospitalizations, and 0.34 deaths per 10,000 population compared with the recommended four-week interval between doses.
Delaying the BNT162b2 Covid-19 vaccine for nine weeks compared with the recommended three-week schedule also avoided an additional 0.60 hospitalizations and 0.32 deaths per 10,000 population. Importantly, however, Galvani and colleagues also found that delaying the second dose of BNT162b2 vaccine did nothing to reduce the number of infections, unless the efficacy of the first dose did not wane with time.
“For Moderna’s two-dose vaccine, we show that a DSD strategy would outperform the recommended interval between doses in terms of reducing the number of hospitalizations and deaths. The maximum benefits would be achieved with a delay of at least nine weeks from the recommended schedule for administering the second dose. A DSD strategy with [BNT162b2] vaccines is comparatively inferior to [mRNA-1273] vaccines, and the delay to achieve maximum benefits depends on the durability of the first-dose efficacy,” concluded Galvani et al.
But the authors also stressed the factors most important to assessing delayed second dose vaccination programs: durability of vaccine efficacy and the ability of vaccines to block transmission, vaccine supply, and other factors such as vaccine-resistant strains. “These are all important considerations in public health decision-making regarding DSD vaccination,” they wrote.
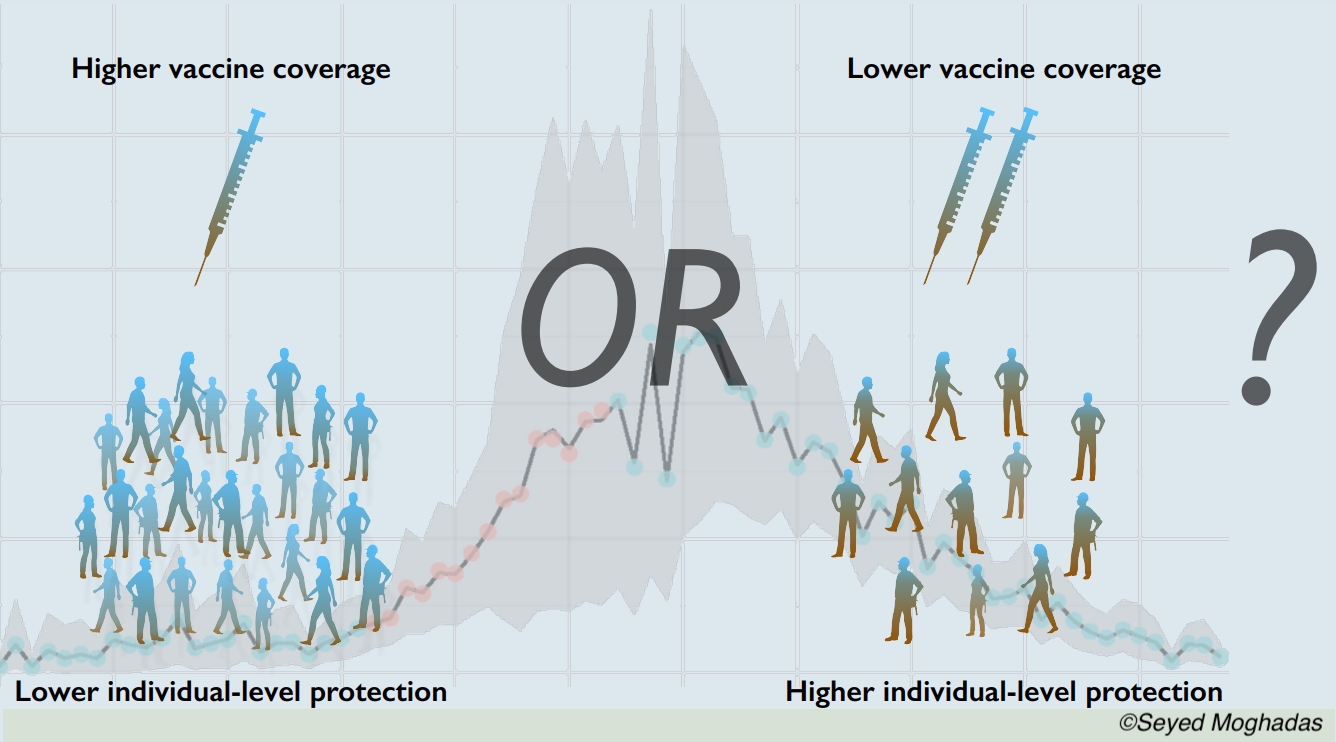
“However, given the relatively high vaccine efficacies estimated after the first dose against severe outcomes (i.e., over 50% in most end points), broader population-level protection would be expected to further reduce the disease burden, even with limited vaccine supplies in the near term. When racing against a burgeoning outbreak, our results show that prioritizing vaccine coverage with rapid distribution of the first dose would be critical to mitigating adverse outcomes and allow the healthcare system to also address non-Covid-19 medical needs of the population. In the case of low incidence, it would still be important to accelerate vaccination with the first dose to protect the maximum number of individuals ahead of any outbreak surge,” concluded Galvani et al.
Regarding the limitations of their study, they wrote: “Our model assumptions were conservative and based on limited empirical evidence available thus far,” adding that no data exist that quantify the decline of vaccine-induced immunity under different schedules of a strategy that delays second dosing. Once these data become available, these results may differ and more study will be warranted, Galvani and colleagues stressed.
- Delaying the second dose of the Moderna and Pfizer-BioNTech Covid-19 vaccines for nine to 15 weeks after the first dose may help avoid more hospitalizations, infections, and death than following the recommended schedules.
- Future studies are needed to quantify the characteristics and durability of vaccine-induced protection after the first dose of the Covid-19 vaccine to better determine the optimal time interval between the two doses.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
This study was funded by the Canadian Institutes of Health Research; São Paulo Research Foundation; the National Institutes of Health, and the National Science Foundation.
The authors reported no further disclosures.
Cat ID: 31
Topic ID: 79,31,730,933,31,926,561,927,151,928,925,934