A new guideline recommends that patients diagnosed with HIV and tuberculosis (TB) receive prompt, coordinated treatment for both conditions. TB is a leading killer among HIV patients, and providing therapy for both illnesses simultaneously may help save lives.
Tuberculosis (TB) is a major public health threat, with 9.6 million cases and 1.5 million associated deaths reported worldwide in 2014. With 13 years passing since the last guidelines on the treatment of TB and the development of a Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the quality of evidence behind recommendations made in guideline, the American Thoracic Society, Infectious Diseases Society of America, and CDC joined forces to develop a new guideline on the treatment of drug-susceptible TB. “Prompt and successful treatment of TB benefits patients as well as the communities in which they live because the infection is transmitted through cough aerosol,” explains Payam Nahid, MD, MPH, lead author of the new guideline.
Highlighting Key Changes
Since the last guideline was published, there has been a substantial increase in the publication of studies focusing on HIV and TB co-infection (HIV-TB), which has helped inform the optimal management of these patients, according to Dr. Nahid. “Perhaps the most significant change in the new guideline relates to the management of patients with HIV,” he says. Specifically, the guideline writing committee addressed whether antiretroviral therapy (ART) initiation should occur during TB treatment or after treatment is completed. The update also addresses the duration of treatment for HIV-TB.
Several recent studies have shown that initiating ART early in TB treatment—as early as 2 weeks, depending on patient characteristics—appears to help reduce mortality risk, progression to other AIDS-defining illnesses, or both. “These studies were the main drivers to recommend strongly that ART should be initiated early during TB treatment,” Dr. Nahid says (Table below). “This should ideally occur within the first 2 weeks for patients with CD4 counts less than 50 or by 8 to 12 weeks for patients with CD4 counts above 50.”
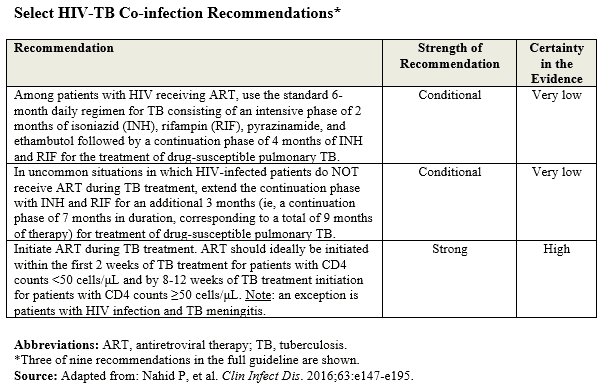
Dr. Nahid says the recommendation for initiating ART during TB treatment is critical to addressing the duration of treatment for HIV-TB. “Going forward, the standard must be to start ART during TB treatment,” he says. “However, in uncommon situations, there may be various reasons why patients may not be initiated on ART.”
According to Dr. Nahid, earlier guidelines recommended that treatment of TB be a 6-month regimen, regardless of HIV serostatus. “After evaluating more recently published literature, it is now recommended that TB treatments be extended to 9 months in HIV-infected patients who don’t receive ART. This appears to reduce risks of TB relapse. It should be noted, however, that this recommendation is only applicable to a very small subset of patients. When ART is initiated during TB treatment—which, as noted is the new standard for all patients, a 6-month regimen is used.”
Case Management
One of the recommendations that Dr. Nahid feels benefited greatly from the GRADE methodology is focused on case management strategies. Case management—which includes patient education and counseling, field and home visits, providing patient reminders and incentives to complete therapies, and use of directly observed therapy (DOT)—has been shown to improve treatment success. The new guideline note that appropriate case management is essential to ensuring effective treatment of all TB patients. In DOT, clinicians watch patients swallow each dose of medication during the 6-month course of therapy.
“The evidence synthesized in the new guidelines on case management strategies offers a better appreciation for the very real value of patient education and counseling, DOT and other interventions to improve treatment success, and to reduce the risk of poor adherence and potentially unfavorable outcomes from treatment,” says Dr. Nahid.
Future Research
Given the burden of TB worldwide, the emergence of drug-resistant TB, and the relatively short list of effective drugs, the development of new drugs and, more importantly, the establishment of more effective drug regimens are crucial needs. Data are also lacking on the treatment of TB in special populations, particularly pregnant women, breastfeeding women, and children. “We need to re-evaluate whether or not future studies can safely include these populations,” says Dr. Nahid. “Without these data, we don’t know the most appropriate way to manage these patient subsets. In the meantime, however, this new guideline includes several new, evidence-based recommendations provided in conjunction with extensively referenced practical information on TB treatment that should be incorporated into current practices by healthcare providers, regardless of their specialty, in order to optimize the care of patients with TB.”
Payim Nahid, MD, MPH, has indicated to Physician’s Weekly that he has no financial interests to disclose.


 PhysWeekly
PhysWeekly