Diabetes is a well-recognized risk factor for cardiovascular disease (CVD), but pre-diabetes is often overlooked in CVD prevention efforts, even though it is more common than diabetes.
And, although treatment guidelines such as the Standards of Medical Care in Diabetes–2019 and its 2021 update—as well as the 2019 joint guideline from the European Society of Cardiology and the European Association for the Study of Diabetes—recognize the importance of pre-diabetes, they “primarily focus on glycemic control and lifestyle management” to reduce the risk of progression to clinical diabetes, overlooking the possibility of either cardiovascular or kidney damage across the glycemic spectrum,” according to Michael C. Honigberg, MD, MPP, of Massachusetts General Hospital.
Honigberg and colleagues used data from the U.K. Biobank to identify 46,911 individuals with pre-diabetes and 12,717 with type 2 diabetes (T2D) and looked at follow-up data over more than a decade. The results of their analysis, which were published online in the Journal of the American College of Cardiology and presented during the Health Promotions Year in Review Session at American College of Cardiology’s virtual meeting ACC.21, found that evidence of heart disease or renal damage may develop prior to an incident diagnosis of T2D.
“Among 6,476 individuals with pre-diabetes who developed ≥1 incident outcome, 1,930 (29.8%) progressed to T2D during follow-up, and only 802 (12.4%) developed T2D prior to an incident diagnosis,” noted researchers.
They excluded from the analysis individuals from the UK Biobank database with type 1 diabetes, CVD, or kidney disease, and assessed the association of T2D, pre-diabetes, and normoglycemia with atherosclerotic CVD (ASCVD), chronic kidney disease (CKD), and heart failure.
“The UK Biobank is a prospective, observational, population-based cohort of more than 500,000 adult residents of the United Kingdom who were 40-69 years old at the time of recruitment between 2006-2010.” All individuals included in the Honigsberg analysis had HbA1c measured at enrollment.
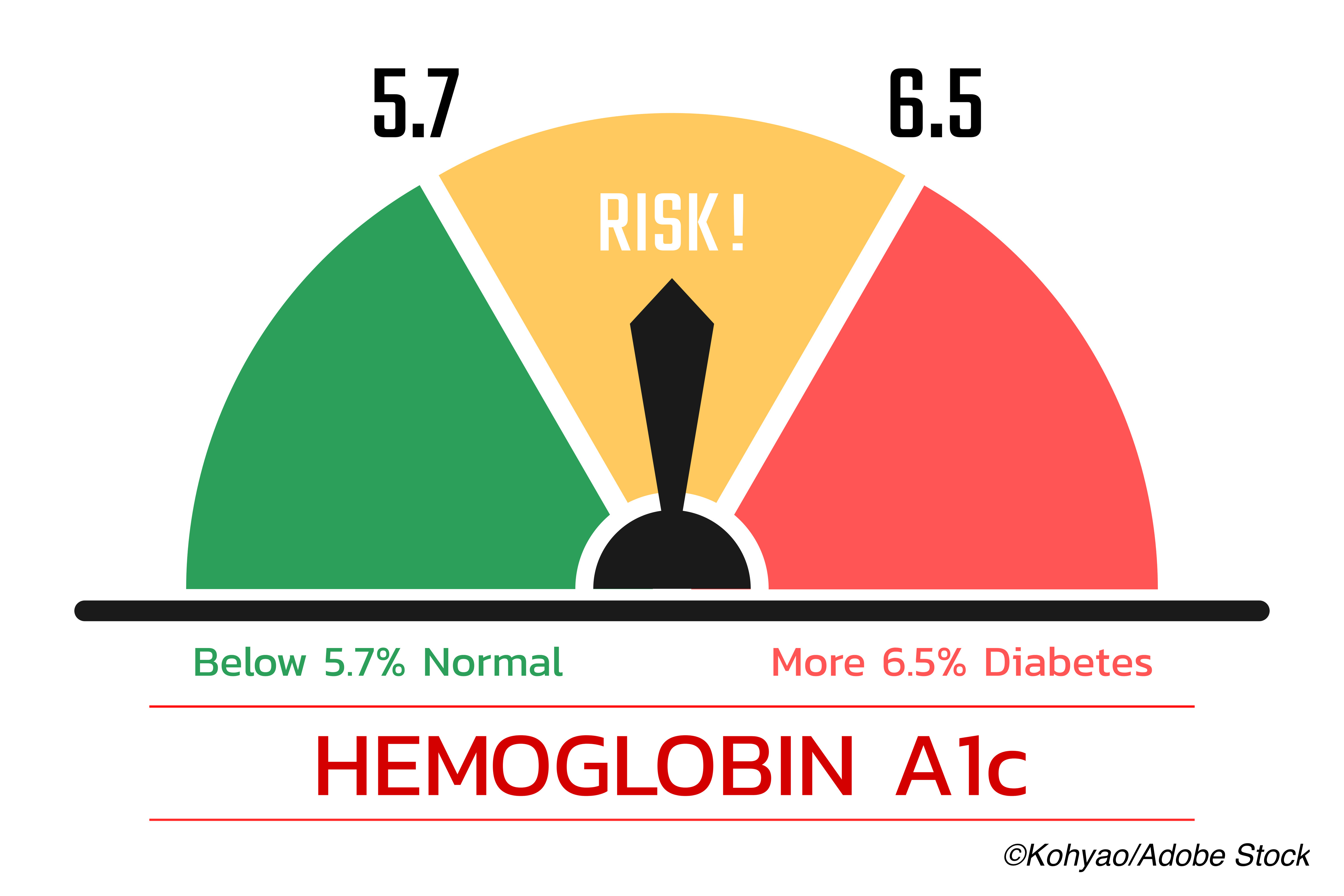
“The primary study exposure was T2D (defined as self-reported T2D, Hb1c ≥6.5%, and/or use of insulin) versus pre-diabetes (defined as no self-reported T2D and HbA1c ≥5.7 and <6.5%, in accordance with the American Diabetes Association definition [18]) versus normoglycemia (defined as no self-reported T2D and HbA1c <5.7%). HbA1c as a continuous variable constituted a secondary exposure,” they wrote.
In all, 336,709 individuals met the study criteria, of whom most (82.3%) had normoglycemia. The mean age at baseline was 65.3 and 55.4% were women. Those with T2D or pre-diabetes were “more likely to be non-White, had lower socioeconomic status, were more like to be smokers or former smokers, have higher BMI, and “higher SBP despite higher rates of antihypertensive medication use, lower HDL cholesterol, higher triglycerides, microalbuminuria and C-reactive protein levels.”
Among the findings:
- 21,769 (7.9%) of individuals with normoglycemia developed at least 1 incident event (ASCVD, CKD, and/or heart failure, as did 6,476 (13.8%) with pre-diabetes, and 3,017 (23.7%) with T2D.
- ASCVD was the most frequent outcome, and occurred in 5.7% of those with normoglycemia, 10.0% of those with pre-diabetes, and 16.8% of those with T2D.
- CAD represented 84.1% of ASCVD diagnoses.
- CKD was diagnosed in 5,473 (2.0%) with normoglycemia, 1,868 (4.0%) with pre-diabetes, and 1,181 (9.3%) with T2D.
- Heart failure was diagnosed in 4,207 (1.5%) with normoglycemia, 1,354 (2.9%) with pre-diabetes, and 660 (5.2%) with T2D.
“In time-to-event models adjusted for age, sex, and race, pre-diabetes versus normoglycemia was associated with increased risk of ASCVD (HR 1.44, 95% CI 1.39-1.49, P<0.001), CKD (HR 1.48, 95% CI 1.40-1.56, P<0.001), and heart failure (HR 1.46, 95% CI 1.38-1.56, P<0.001). After complete covariate adjustment, pre-diabetes remained independently associated with ASCVD (HR 1.11, 95% 1.08-1.15, P<0.001) and CKD (HR 1.08, 95% CI 1.02-1.14, P=0.009) and nominally significantly associated with heart failure (HR 1.07, 95% CI 1.01-1.14, P=0.03). As expected, T2D was independently associated with all outcomes. Pre-diabetes, T2D, and each unit change in HbA1c appeared to associate most strongly with peripheral artery disease among ASCVD subtypes.”
Compared to normoglycemia, pre-diabetes was also independently associated with all-cause mortality (HR 1.10, 95% CI 1.05-1.14, P <0.001). Honigberg wrote that after “full covariate adjustment, the risk of all-cause mortality appeared to increase beginning at HbA1c levels above 6.2%.”
Honigsberg et al listed four clinical implications of their findings:
- HbA1c should be considered a continuous measure of risk, rather than “dichotomized as ≥6.5% versus <6.5%.”
- A first ASCVD or CKD event signals “accumulating multimorbidity.”
- Patients with pre-diabetes have an elevated risk of cardiovascular or kidney disease even before progression to T2D.
- Consider pre-diabetes as a criteria for inclusion in trials recruiting high risk ASCVD individuals.
There are number of limitations that should be considered when interpreting the results: the UK Biobank database represents generally healthy individuals and this healthy participant bias may result in underestimation of population attributable risks of dysglycemia. Additionally, the study was not powered to test “glycemic relationships in specific non-White subgroups and routine follow-up HbA1c was not available for analysis.”
-
In this analsysis, pre-diabetes and T2D were associated with a substantial gradient of cardiovascular and renal risk across HbA1c levels below the threshold for diabetes.
-
Be aware that individuals with pre-diabetes may develop measurable cardiovascular and kidney disease prior to progressing to T2D.
Peggy Peck, Editor-in-Chief, BreakingMED™
Honigberg is supported by a grant from the National Heart, Lung, and Blood Institute.
Coauthors of the study reported grant support, consultant or advisory board fees from Amgen, Apple, Boston Scientific, American Regent, AstraZeneca, Bayer, AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Relypsa, and Galmed.
Cat ID: 12
Topic ID: 76,12,730,305,446,914,12,187,307,192,669,918,925,168