Previous research has shown that hospitalized patients who experience an acute kidney injury (AKI) have higher in-hospital mortality rates, are more susceptible to adverse long-term outcomes, and accrue significant healthcare costs. “Studies indicate that about 10% to 15% of patients undergoing PCI will have an AKI,” says Roxana Mehran, MD. “These patients have a higher mortality risk than those who do not suffer such injuries.”
While there is a plethora of data regarding the connection between AKI and PCI, the incidence and impact of AKI after CABG surgery have not been well established in clinical research. This is largely the result of variations in study populations and disparities in how AKI is defined.
“For the CABG surgery population, the risk of developing an AKI is further compounded by exposure to iodinated contrast from diagnostic coronary angiography,” explains Dr. Mehran. She notes that CABG has been associated with higher risks for AKI due to pathophysiological processes, such as systemic hypotension, the diversion of renal blood flow, and inflammatory mediators, among other processes.
Taking a Closer Look
Data are needed to quantify the incidence of AKI in patients treated with CABG for acute coronary syndromes (ACS) and to establish the short- and long-term risk of adverse outcomes that are associated with AKI. For a study published in the American Heart Journal, Dr. Mehran and colleagues used pooled data to quantify the incidence of AKI in patients treated with CABG for ACS as well as associated risks.
The data used in the study came from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) and Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trials, which were multicenter studies examining the safety and efficacy of bivalirudin in patients presenting with ACS. The HORIZONS-AMI trial looked at more than 3,600 patients presenting with STEMI within 12 hours of symptom onset. The ACUITY trial involved nearly 14,000 patients with moderate- to high-risk non–STEMI ACS who received PCI between August 2003 and December 2005.
Of the more than 17,000 patients enrolled in these trials, Dr. Mehran and colleagues found that about 8% underwent CABG as their principal treatment after coronary angiography. The authors then assessed rates of death, myocardial infarction, and ischemia-driven target vessel revascularization at 1 month and 1 year. AKI was defined as a rise in creatinine levels of 0.5 mg/dL or more (or more than 25%) from baseline at initial angiography.
Highlighting Key Findings
Patients who developed AKI were more likely to be older, of non-white heritage, and to have comorbidities like hypertension and diabetes. Several well-recognized predictors of AKI—including contrast volume, existing renal impairment, and female sex—were not associated with the development of AKI in the analysis. This may be due to more stringent preventative measures being used in these at-risk patients to mitigate risks of AKI. The authors added that AKI patients had lower hematocrit and hemoglobin levels, which are known modifiable risk factors for AKI.
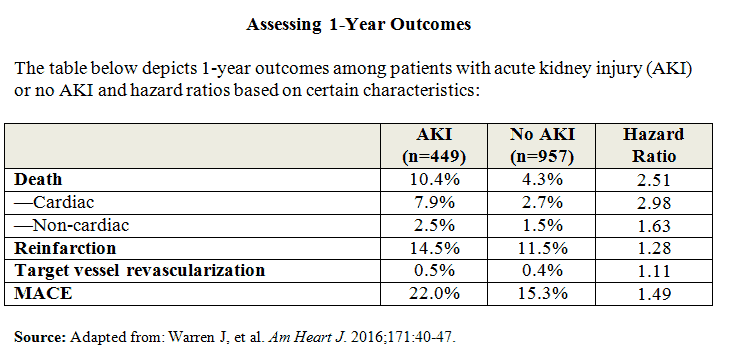
“About 32% of patients who were treated with CABG had an AKI occur during their hospital admission,” says Dr. Mehran. “At 1 month and 1 year after these patients were discharged, the rates of all-cause mortality and cardiac death were significantly higher in patients who had developed AKI.” The 1-year mortality rate for patients who developed AKI treated with CABG was nearly 18%. Similarly, the composite outcome of major adverse cardiac events (MACE) was higher in these patients at 1 month and the trend persisted at 1 year (Table below).
Important Implications
Results of the analysis suggest that AKI is an independent predictor of mortality—both overall and cardiac-related—and MACE at both 1 month and 1 year in patients treated with CABG. “Our findings are important because such a large number of patients undergoing CABG for ACS appear to develop AKI,” says Dr. Mehran. “In addition, the development of AKI appears to be a strong independent predictor for mortality and MACE in both the short and long term after patients receive CABG.”
Experts have recommended several prevention strategies to reduce the risk of contrast-induced AKI, including hydration, correction of baseline anemia, the use of N-acetyl cysteine, and statin therapy. “Given the significant risks of AKI, there is an urgent need to identify at-risk populations and implement appropriate risk-reducing strategies,” Dr. Mehran says. “Clinicians need to be vigilant about the significant mortality risk associated with AKI and prepare patients’ kidneys by taking preventive measures and keeping a close eye on creatinine levels.”
The study notes that patients who are referred for CABG over PCI are generally older and, thus, have a higher incidence of existing renal impairment and more advanced heart disease. With older patients already at higher risk for AKI and the increasing trend of an aging population in the United States, Dr. Mehran says the development of preventive strategies for these injuries is critical to ensuring better patient outcomes.
Roxana Mehran, MD, has indicated to Physician’s Weekly that she has received institutional research grant support from The Medicines Company, Bristol-Myers Squibb/Sanofi, Eli Lilly and Company/Daiichi-Sankyo, Regado Biosciences, and STENTYS. She has also received consulting fees from Abbott Vascular, AstraZeneca, Boston Scientific, Covidien, CSL Behring, Janssen Pharmaceuticals, Maya Medical, and Merck & Co. In addition, she has served as an advisory board member of Covidien, Janssen Pharmaceuticals, Merck, Sanofi, and Endothelix Inc., and is an equity shareholder of Endothelix Inc.


 PhysWeekly
PhysWeekly