Recent Alzheimer’s disease research has poised the field for a watershed period in diagnosis and treatment, which may begin as early as this year.
This shifting clinical landscape in Alzheimer’s research includes blood tests to identify possible biomarkers, the first FDA-approved tau PET ligand, and the possibility of the first disease-modifying therapy — the amyloid beta (Aβ)-targeted monoclonal antibody aducanumab — that may be approved by the FDA this year. Moreover, the treatment pipeline continues to see robust research activity, with both traditional and novel targets in development and clinical trials.
Amyloid, Tau, and Other Targets
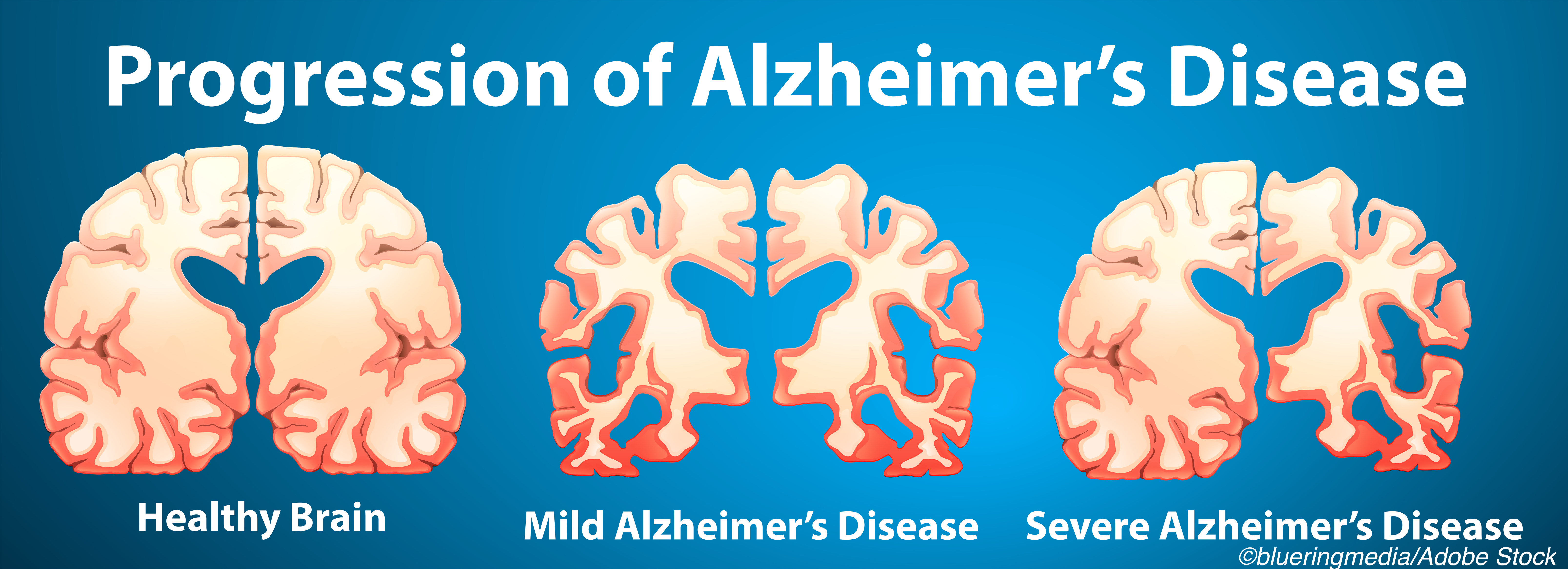
The amyloid cascade hypothesis, proposed in 1992, considered deposits of extracellular amyloid beta (Aβ) protein (plaques) in the brain as the first step in Alzheimer’s pathology, followed by intracellular tau protein deposits (tangles) and neural and synaptic loss. Decades after these initial events, Alzheimer’s cognitive symptoms emerge.
Both types of deposits consist of insoluble fibrils of normally soluble constituents and involve prion-like misfolded templates and trans-synaptic spread. Contemporary understanding of Alzheimer’s has evolved since then and has extended the original postulates, while retaining Aβ and tau protein deposition as key pathologic findings.
Pathologic Aβ deposits can occur as both extracellular plaques (Alzheimer’s disease) and in the walls of small- and medium-sized cerebral arteries (cerebral amyloid angiopathy) when imbalance between production and clearance occurs. Importantly, Aβ accumulation begins 10 to 20 years before cognitive decline is evident and correlates poorly in both burden and location with cognitive decline.
Phosphorylated species of tau are present at low levels shortly after Aβ begins to accumulate, before neurofibrillary tangles are detectable and well before symptoms emerge. Tauopathy distribution better correlates with cognitive findings than Aβ.
Other factors that may play a role in Alzheimer’s disease, like inflammation or infection, also are being investigated. Recently, the TREM2 immunoreceptor on central nervous system resident macrophages (microglia) has been studied as part of the immune response to Aβ, with both harmful and salutary effects on Aβ and tau pathology. Gut microbiome modulation of microglial development and function may play a role. Aβ itself is part of an immune response to bacteria, fungus, and viral agents, which may induce Aβ fibrillization and begin the amyloid cascade — the antimicrobial protection hypothesis, with recent focus on herpesvirus and correlations between periodontal infectious toxins (gingipains) and tau pathology in Alzheimer’s patients.
Other links and risk factors include genetics and multiple environmental exposures. For late-onset (after age 65) disease, which accounts for 95% of Alzheimer’s cases, the apolipoprotein E4 (APOE4) allele is the strongest genetic risk factor. APOE4 homozygotes are at the highest risk, while carrying an APOE2 allele is associated with lower risk.
Recent research has reported associations of unclear causal direction for cognitive decline with stress, sleep abnormalities, and multiple factors including diabetes, hormones and menopause, cardiovascular risks and cerebral amyloid angiopathy, and vitamin D deficiency. The relationship between hypertension and Alzheimer’s disease likely varies over the lifespan, with midlife hypertension appearing to convey the most risk.
Researchers have found connections between air pollution and incident Alzheimer’s disease. Lifestyle factors including education, exercise, and cognitive activity in enriched environments have been reviewed recently with an emphasis on anti-inflammatory effects with respect to potential benefits. Resistance and resilience — not developing Alzheimer’s pathology with age, or maintaining normal cognition in spite of Alzheimer’s pathology — are of particular research interest.
Clinical Diagnosis
Clinical diagnosis results from deficits in two or more cognitive domains in the absence of treatable mimics, which should be screened for with blood tests, history and physical examination, and structural imaging (CT or MRI to rule out resectable tumor or normal pressure hydrocephalus, for example), per guidelines. Additional evaluations for other associated or mimicking conditions and for ongoing vascular risk factor management is individualized.
Cognitive deficits are typically documented and followed with short screening tools, including the Mini-Mental State Examination (MMSE) and the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog). Cognitive change with preserved daily functional ability is often considered mild cognitive impairment (MCI). Some patients with MCI may have another form of dementia or a dementia mimic.
In 2020, an Alzheimer’s blood test became available for clinical use, regulated under the CMS Clinical Laboratory Improvement Amendments (CLIA) program. In a study, the test — known as PrecivityAD — correctly identified brain amyloid plaque status (which was determined by amyloid PET scans) in 86% of older adults who had subjective cognitive impairment or dementia. PrecivityAD is not a standalone test for Alzheimer’s disease, but can help physicians in the evaluation process, according to its manufacturer.
Confirming Diagnosis
While clinical diagnosis and staging remain important in many settings, treatment development and trial research often focuses on biologic diagnosis of Alzheimer’s disease. A 2018 update to the National Institute on Aging and Alzheimer’s Association research framework reaffirmed the 2011 commitment to grouping imaging and fluids biomarkers by the pathologic process each measures in Aβ, tau, or neurodegeneration—the AT(N) system.
AT(N) data can be derived from autopsy, biopsy, and cerebrospinal fluid (CSF) measures, as well as imaging and blood tests. Structural MRI assesses overall anatomy and regional atrophy. PET scans can image hypometabolism based on labeled glucose ligand uptake (FDG PET) as well as Aβ and tau using specific tracer ligands (florbetapir for Aβ or flortaucipir, which became the first tau PET ligand to earn FDA approval in 2020, for example).
Perhaps the greatest game-changer in Alzheimer’s trials may be blood tests for amyloid or tau. “Therapeutic or secondary prevention clinical trials of amyloid- or tau-specific therapeutics want to enroll individuals who are amyloid- or tau-positive,” noted Michelle Mielke, PhD, of the Mayo Clinic in Rochester, Minnesota.
“Currently, to establish whether someone has amyloid and tau pathology, a lumbar puncture for the collection of CSF or amyloid or tau PET is needed,” Mielke told BreakingMED. “Both are expensive and invasive. Blood-based biomarkers of amyloid and tau pathology are much less invasive, more feasible, and will greatly reduce the costs of identifying individuals for clinical trials.”
Blood tests that screen for amyloid-beta could cut the need for PET scans by 50% in asymptomatic Alzheimer’s research trials, a U.K. study recently suggested. Blood-based tests being developed for phosphorylated tau (p-tau) 217 and 181, which are present early in Alzheimer’s disease, have reported very high accuracy — greater than 90% — for predicting tau PET scan positivity.
Treatments: Now and Future Possibilities
Currently approved Alzheimer’s treatments — which treat Alzheimer’s symptoms only and are not disease-modifying — are three cholinesterase inhibitors, donepezil (Aricept), rivastigmine (Exelon), and galantamine (Razadyne); the NMDA receptor modulator memantine (Namenda); and a combination treatment of memantine and donepezil (Namzaria). These drugs have been extensively reviewed.
Investigational disease-modifying treatments for Alzheimer’s disease have targeted Aβ or tau or have taken other approaches. These include monoclonal antibodies to Aβ, which have led to multiple trial failures in the past.
Some of these anti-amyloid drugs, like solanezumab and BAN2401 are being re-evaluated, this time in asymptomatic Alzheimer’s patients. Promising cognitive results also were recently announced for another investigational monoclonal antibody, donanemab, with peer-reviewed data from its phase II study expected later this year.
In June, the FDA is expected to decide whether to approve aducanumab, a monoclonal antibody targeting Aβ that showed mixed results in two phase III trials, opening the door to public controversy about the drug’s effectiveness. If approved, aducanumab will be the first disease-modifying therapy available to Alzheimer’s patients.
Last November, the FDA’s advisory committee recommended against approving aducanumab, saying there was not enough evidence in the drug’s two phase III trials to decide. But not everyone in the Alzheimer’s community agreed with that recommendation.
“From my perspective, the aducanumab trial outcomes demonstrate that patients treated with the higher dose for at least 10 months exhibit both removal of amyloid on PET scanning and slowing of cognitive decline as measured by standard trial instruments,” said Jeffrey Cummings, MD, ScD, of University of Nevada, Las Vegas, who was not part of the FDA advisory committee.
Biomarkers linked to amyloid changed in the trials, adding to evidence of efficacy, Cummings told BreakingMED. “The difference between the two trials can be accounted for by differences in dose and duration of treatment,” he noted.
“Approval of aducanumab will stimulate more innovation and more investment in Alzheimer’s drug development,” Cummings added. “Most importantly, aducanumab, if approved, will provide a new therapy for mildly affected patients and will give all patients and caregivers hope for new therapeutic breakthroughs.”
In an analysis published in Alzheimer’s & Dementia, however, David Knopman, MD, of Mayo Clinic in Rochester, Minnesota, and colleagues argued that the aduncanumab trials failed to demonstrate efficacy. “Biomarker data were consistent with target engagement, but no evidence was presented to correlate biomarker changes to cognitive benefits,” they noted.
“Our analysis supports the conduct of a third, phase III trial with high-dose aducanumab,” Knopman and co-authors wrote. “Aducanumab’s efficacy as a treatment for the cognitive dysfunction in Alzheimer’s disease cannot be proven by clinical trials with divergent outcomes.”
Aβ production may be targeted by inhibiting BACE (β-site amyloid precursor protein cleaving enzyme). Multiple BACE inhibitors have failed phase III trials; some, like atabecestat, led to worse cognitive functioning and neuropsychiatric events that appeared to be temporary.
Tau reduction strategies also include tau vaccines AADvac-1 and ACI-35.030, plus passive immunization treatments in development.
Other approaches to treating Alzheimer’s include trials involving gingipain inhibition and/or young blood. Studies of drugs that potentially could be repurposed as Alzheimer’s therapy include lithium and a low-dose form of levitiracetam.
Evaluation is also ongoing for strategies addressing neuronal signaling underlying plasticity and memory function and protein misfolding; sumifilam, a compound that binds to filamin A, is scheduled to enter phase III study this year. An ongoing phase II trial is evaluating the anti-amyloid combination of tauroursodeoxycholic acid and sodium phenylbutyrate (AMX0035), which also is being studied as a possible treatment for ALS.
Managing Alzheimer’s Today
For now, medical treatment for Alzheimer’s disease remains symptomatic. An important treatment question in the disease course is whether to medically treat issues like sleep disturbances or psychosis. In 2020, the FDA approved an update to the prescribing information for suvorexant (Belsomra), an orexin receptor antagonist, to include findings about treating insomnia in patients with mild-to-moderate Alzheimer’s disease.
Using antipsychotics for behavioral changes in Alzheimer’s disease is problematic; the drugs have included black box warnings for death. The antipsychotic pimavanserin (Nuplazid), approved for hallucinations and delusions in Parkinson’s disease, is being reviewed by the FDA for dementia-related psychosis in Alzheimer’s patients. Other strategies including use of agents from various classes in a graded, systematic approach if non-medical treatment options are insufficient.
Realistic expectations are important globally in management, with specific implications for all medications and treatment goals for both Alzheimer’s disease and comorbidities. Trade-offs are frequent and at times can be distressing to patients, caregivers, and clinicians.
Pitfalls in medical treatment of Alzheimer’s disease often include the failure to recognize or address the effects of polypharmacy, particularly medications used for non-cognitive treatments (such as beta blockers, pain medication, and many others) and comorbidities. Over-treatment in place of investigating potential non-dementia sources of possibly transient decline (such as infections, or comorbidities like depression or anxiety) also can be a problem.
Treating and managing Alzheimer’s disease often includes assessing risk factors like vision and hearing, encouraging fall risk precautions, providing appropriate referrals including to speech, occupational, or physical therapy as needed, and discussing home safety for the Alzheimer’s patient and others, including driving or harms that may occur from guns in the household or wandering.
The goal of establishing safe, non-stressful physical and cognitive environments for Alzheimer’s patients may require educating caregivers and family members. This is especially true during the Covid-19 public health emergency.
A recent analysis of 62 million electronic health records in the U.S. showed that people with dementia had twice the risk of SARS-CoV-2 infection as other adults, with Black patients carrying especially high risk. Alzheimer’s patients had an odds ratio of 1.86 of developing Covid-19, wrote Rong Xu, PhD, of Case Western Reserve University School of Medicine in Cleveland, and co-authors, in Alzheimer’s & Dementia.
The study found that 6-month mortality risk for people with dementia and Covid-19 was 21%, and hospitalization risk was 59%. Findings were observational, not causal, and need to be replicated and compared with other databases and patient registries, Xu and colleagues emphasized. Nonetheless, “these findings highlight the need to protect patients with dementia as part of the strategy to control the Covid‐19 pandemic,” they said.
-
This activity disccusses investigational therapies not yet FDA-approved for clinical use.
-
The Alzheimer’s research pipeline includes blood tests to identify possible biomarkers, as well as the the possibility of the first disease-modifying therapy.
Paul Smyth, MD, Contributing Writer, BreakingMED™
Mielke disclosed relationships with Biogen and Brain Protection Company.
Cummings has consulted for Acadia, Alkahest, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Cassava, Cerecin, Cerevel, Cortexyme, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, Jazz, Karuna, Merck, Novo Nordisk,Otsuka, ReMYND, Resverlogix, Roche, Samumed, Samus, Signant Health, Sunovion, Suven,United Neuroscience, and Unlearn AI.
Knopman serves on a data safety monitoring board for the DIAN study. He serves on a data safety monitoring board for a tau therapeutic for Biogen, but receives no personal compensation and is a site investigator in the Biogen aducanumab trials. He is an investigator in a clinical trials sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences but receives no personal compensation. He receives research support from the NIH. He is a member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee and was recused from the November 6, 2020 aducanumab advisory committee meeting.
Xu has no financial interests to disclose.
Cat ID: 130
Topic ID: 82,130,282,485,494,730,130,33,192,255,925


