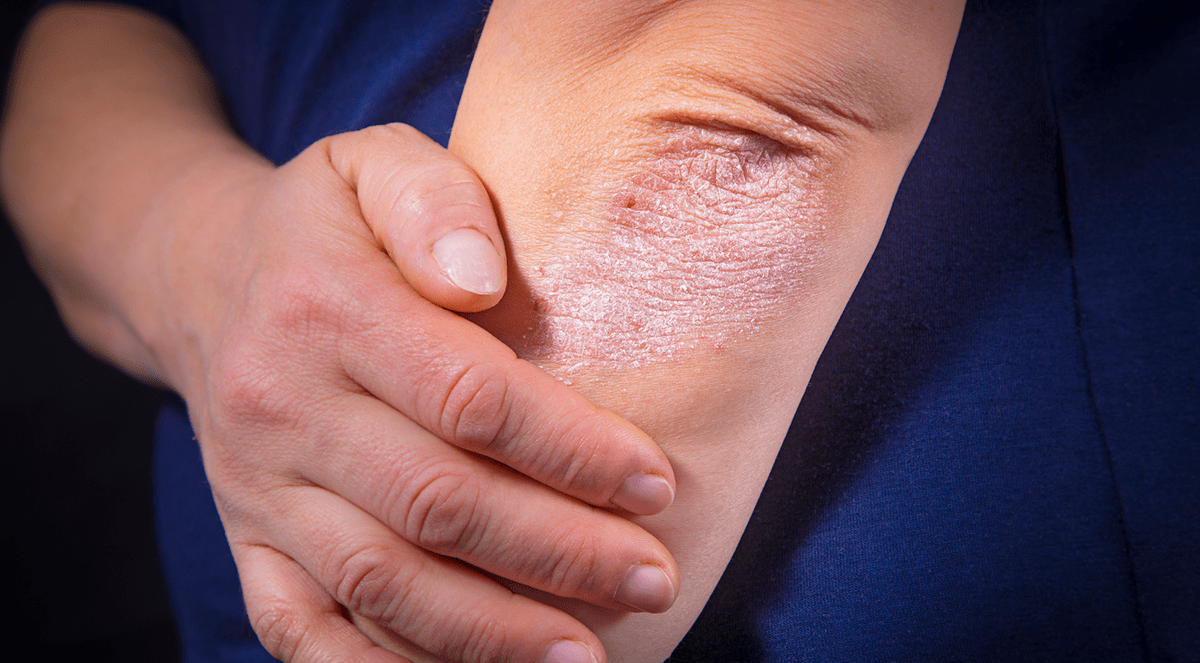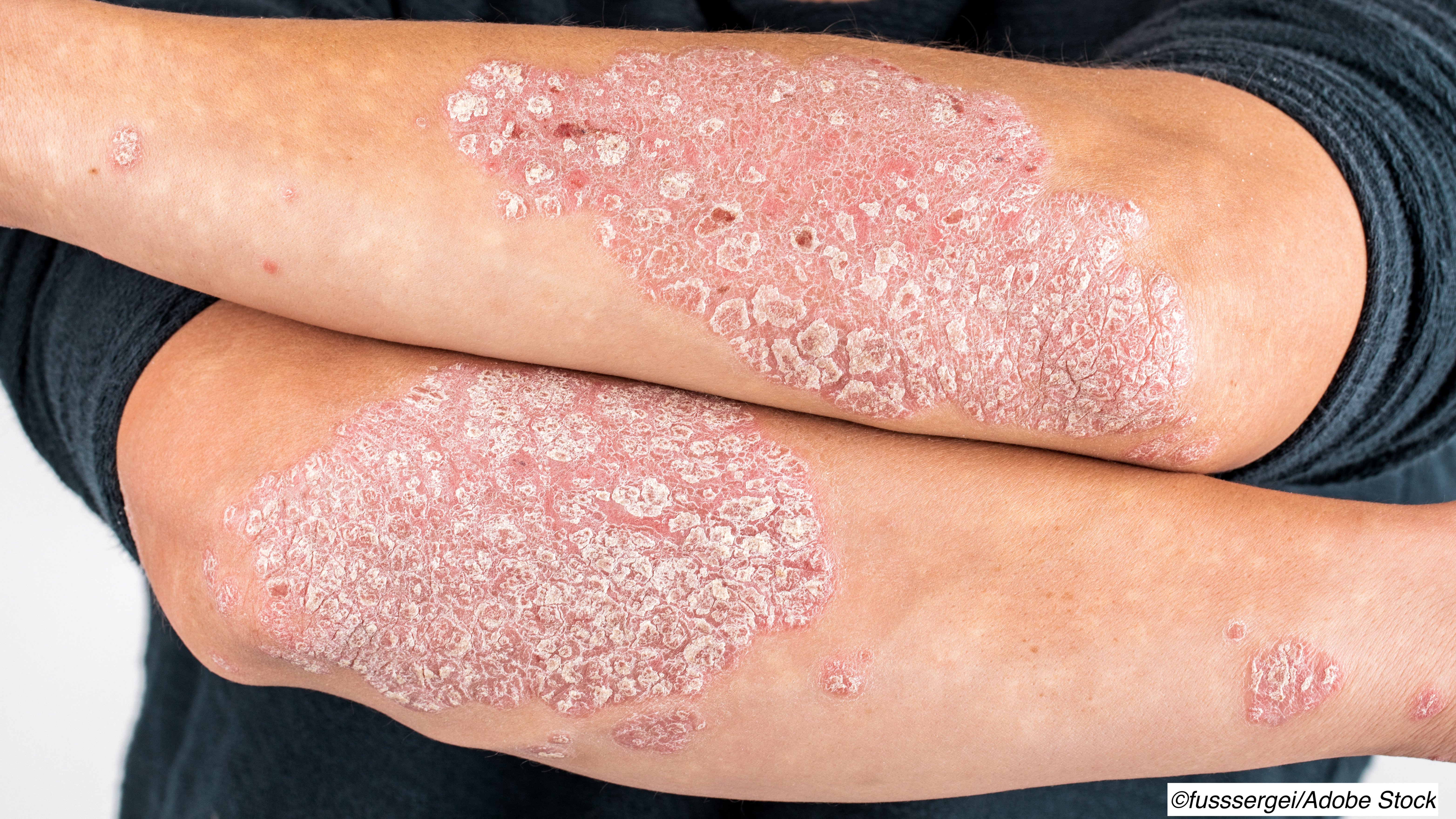
In a pair of trials of patients with moderate to severe plaque psoriasis comparing a novel monoclonal IgG1 antibody to established plaque psoriasis adalimumab (Humira) or secukinumab (Cosentyx), the investigational agent met the primary endpoints—confirming it as both noninferior and superior.
The studies, which are part of a series of phase III trials pitting bimekizumab against established therapies, were reported at the American College of Dermatology virtual meeting and simultaneously published online in The New England Journal of Medicine.
Richard B. Warren, MD, PhD, of the Dermatology Centre, Salford Royal NHS Foundation Trust, Manchester NIHR Biomedical Research Centre, University of Manchester, Manchester, England, and colleagues compared three treatment regimens:
- Bimekizumab 320 mg subcutaneous every 4 weeks for 56 weeks (n= 158).
- Bimekizumab 320 mg every 4 weeks for 16, then every 8 weeks for weeks 16 to 56 (n=161).
- Subcutaneous adalimumab at 40 mg every 2 weeks for 24 weeks, followed by bimekizumab 320 mg every 4 weeks through 56 weeks (n=159).
In the BE SURE trial with adalimumab as a comparator, “bimekizumab was noninferior and superior to adalimumab through 16 weeks in reducing symptoms and signs of plaque psoriasis but was associated with a higher frequency of oral candidiasis and diarrhea,” Warren et al reported.
The primary endpoints of the trial were a reduction of 90% or greater in the Psoriasis Area and Severity Index (PSAI) from baseline and an Investigator’s Global Assessment (IGA) score of 0 or 1—indicating clear or almost clear skin. At baseline, the mean PSAI score was 19.8.
“At week 16, a total of 275 of 319 patients (86.2%) who received bimekizumab (both dose groups combined) and 75 of 159 (47.2%) who received adalimumab had a PASI 90 response (adjusted risk difference, 39.3 percentage points; 95% confidence interval [CI], 30.9-47.7; P<0.001 for noninferiority and superiority). A total of 272 of 319 patients (85.3%) who received bimekizumab and 91 of 159 (57.2%) who received adalimumab had an IGA score of 0 or 1 (adjusted risk difference, 28.2 percentage points; 95% CI, 19.7-36.7; P<0.001 for noninferiority and superiority),” they wrote.
The second trial, BE RADIANT, which used secukinumab as comparator, was reported by a team lead by Kristian Reich, MD, PhD of the Center for Translational Research in Inflammatory Skin Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
In that study, patients with moderate to severe plaque psoriasis were randomized to “bimekizumab at a dose of 320 mg every 4 weeks or secukinumab at a dose of 300 mg weekly to week 4, followed by every 4 weeks to week 48. At week 16, patients receiving bimekizumab underwent rerandomization, in a 1:2 ratio, to receive maintenance dosing every 4 weeks or every 8 weeks to week 48.” They assigned 373 patients to bimekizumab and 370 to secukinumab. The primary endpoint was a 100% reduction in PSAI score from baseline to week 16.
“The primary analysis was first tested for the noninferiority of bimekizumab to secukinumab at a margin of −10 percentage points and then tested for superiority.”
Among the findings:
- At week 16, 61.7% of bimekizumab patients achieved 100% reduction from baseline PASI score versus 48.9% of secukinumab patients.
- Bimekizumab met the margin for both noninferiority and superiority (P <0.001).
“At week 48, a total of 250 patients (67.0%) treated with bimekizumab had a PASI 100 response, as compared with 171 patients (46.2%) treated with secukinumab (adjusted risk difference, 20.9 percentage points; 95% CI, 14.1-27.7; P<0.001). At the week 4 time point, 265 patients (71.0%) in the bimekizumab group had 75% or greater reduction from baseline in the PASI score, as compared with 175 patients (47.3%) in the secukinumab group (adjusted risk difference, 23.7; 95% CI, 17.0-30.4; P<0.001),” Reich and colleagues wrote.
In both trials, treatment with bimekizumab was associated with greater toxicity candidiasis—reported by 19.3% of bimekizumab patients in the BE RADIANT trial and in BE SURE “upper respiratory tract infections, oral candidiasis (predominantly mild or moderate as recorded by the investigator), hypertension, and diarrhea.”
Limitations of the studies include the fact that neither trial had a placebo group, and more than 90% of patients in each study were white.
Psoriasis is a lifelong disease and investigators from both trials noted that longer studies are needed to confirm the durable benefit and safety of bimekizumab.
Both the Food and Drug Administration and the European Medicines Agency are currently reviewing marketing applications for bimekizumab for treatment of adults with moderate to severe plaque psoriasis.
-
In a pair of trials against active comparators, bimekizumab proved noninferioirty and superiority for treatment of adults with moderate to severe plaque psoriasis.
-
Be aware that bimekizumab is not yet approved for clinical use.
Peggy Peck, Editor-in-Chief, BreakingMED™
The BE SURE and BE RADIANT trials were funded by UCB Pharma.
Warren reported grants and personal fees from AbbVie, grants and personal fees from Almirall, personal fees from Amgen, personal fees from Arena, personal fees from Avillion, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers Squibb, personal fees from Celgene, grants and personal fees from Eli Lilly, grants and personal fees from Janssen, grants and personal fees from LEO Pharma, grants and personal fees from Novartis, personal fees from Pfizer, personal fees from Sanofi, grants and personal fees from UCB Pharma, personal fees from Astellas, personal fees from GSK, outside the submitted work.
Reich reported grants and personal fees from AbbVie, grants and personal fees from Affibody, grants and personal fees from Almirall, personal fees from Amgen, grants and personal fees from Biogen-Idec, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Celgene, grants and personal fees from Covagen, grants and personal fees from Eli Lilly, grants and personal fees from Forward Pharma, grants and personal fees from Galderma, personal fees from GSK, grants and personal fees from Janssen-Cilag, grants and personal fees from Kyowa Kirin, grants and personal fees from LEO Pharma, grants and personal fees from Medac, grants and personal fees from MSD, grants and personal fees from Novartis, grants and personal fees from Ocean Pharma, grants and personal fees from Pfizer, personal fees from Samsung Bioepis, grants and personal fees from Sanofi, grants and personal fees from Sun Pharma, grants and personal fees from Takeda, grants and personal fees from UCB Pharma, personal fees from Valeant, grants from Fresenius Medical Care, grants from Galapagos, grants from Miltenyi, grants from XBiotech, personal fees from Xenoport, grants and personal fees from Bristol Myers Squibb, personal fees from Sandoz, outside the submitted work .
Cat ID: 10
Topic ID: 75,10,730,10,105,192,919,925