Although thrombosis with thrombocytopenia has been reported after vaccination with adenovirus vector vaccines for Covid-19, researchers have now reported the first case of vaccine-induced thrombosis with thrombocytopenia (VITT) or thrombocytopenia with thrombosis syndrome (TTS) in a patient receiving the mRNA-1273 vaccine (Moderna).
“Thrombosis with thrombocytopenia has been reported after vaccination against SARS-CoV-2 with 2 vaccines based on recombinant adenovirus vectors—the ChAdOx1 vaccine from AstraZeneca and the Ad26.COV2.S vaccine from Johnson & Johnson/Janssen. This syndrome is similar to heparin-induced thrombocytopenia (HIT), and it is known as vaccine-induced thrombosis with thrombocytopenia (VITT) or thrombocytopenia with thrombosis syndrome (TTS). To our knowledge, there are no reports of VITT or TTS after a SARS-CoV-2 vaccine based on messenger RNA (mRNA) technology,” wrote Robert B. Kaplan, MD, of Allegheny Health Network, Pittsburgh, Pennsylvania, and colleagues in a letter published in Annals of Internal Medicine.
They detailed a case of a 65-year-old male patient who presented with bilateral lower-extremity discomfort, headaches, and dyspnea after his second vaccine dose of the mRNA-1273 vaccine 10 days prior to symptom onset.
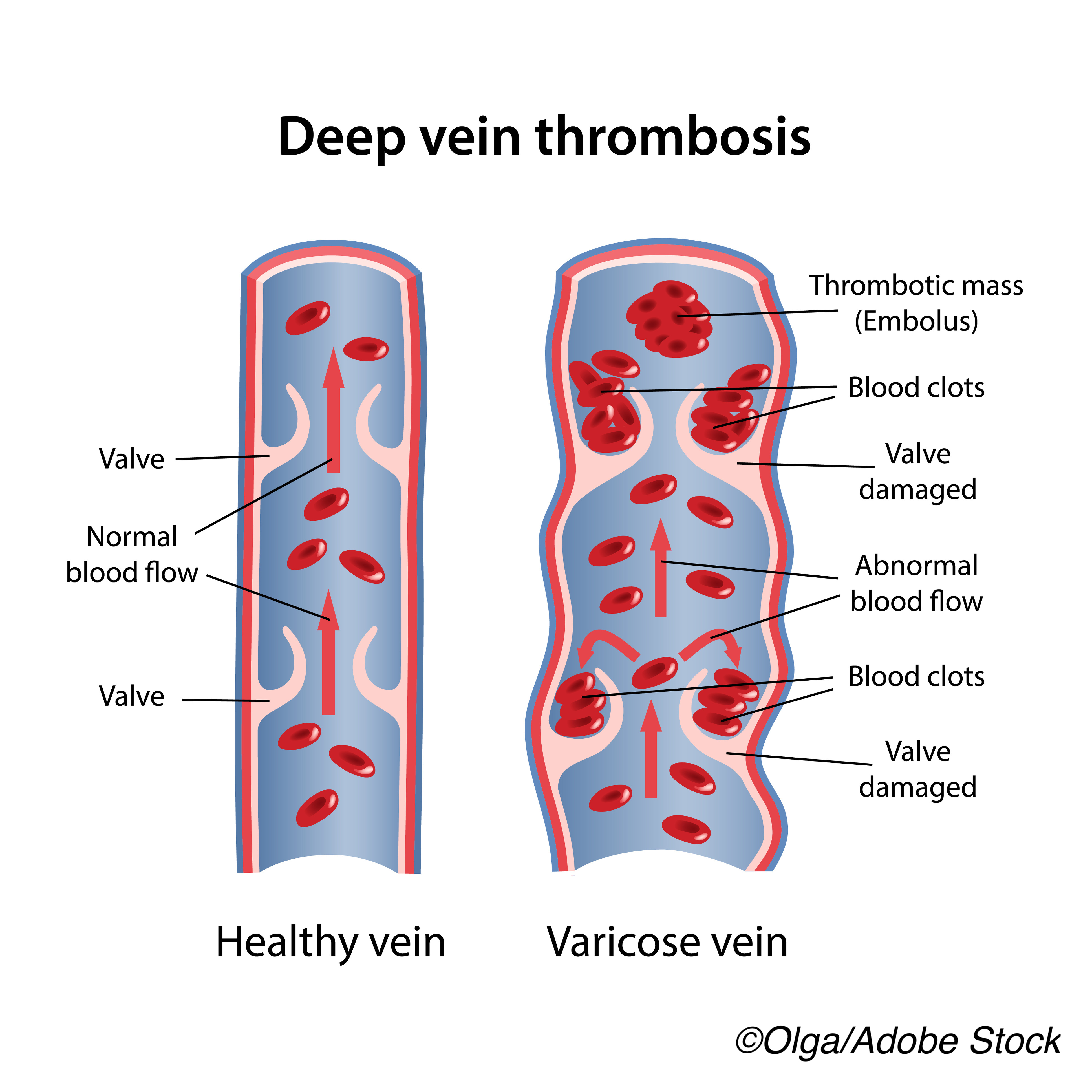
Large, bilateral, acute pulmonary emboli were present on CT angiogram, as was right ventricular strain. Upon Doppler imaging of the legs, researchers observed bilateral acute venous thromboses. The patient was diagnosed with severe thrombocytopenia (14 x 109 cells/L), contraindicating systemic anticoagulative therapy.
The patient received two doses of IV immunoglobulin, followed by 40 mg dexamethasone for 4 days. After platelet transfusions, his platelet count climbed to 69,000 x 109 cells/L, and unfractionated heparin therapy was initiated. Heparin was withdrawn 3 days later, after the patient developed an acute gluteal hematoma.
“The thrombocytopenia persisted, and we evaluated him for HIT. An enzyme-linked immunosorbent assay for anti–platelet factor 4/heparin IgG was strongly positive (2.669 optical density; reference value ˂0.399 optical density),” wrote Kaplan et al.
Head and neck CT angiogram revealed cerebral venous sinus thrombosis; lower extremity DVT and new upper-extremity DVT were also manifest. The patient was treated with bivalirudin but continued to deteriorate. Compartment syndrome of the lower extremities occurred and required emergent bilateral fasciotomies. Methicillin-sensitive Staphylococcus aureus was present in blood cultures, and treatment with vancomycin was initiated.
The patient continued to deteriorate and died after compassionate extubation.
“After his death, serum collected during admission, but before he received heparin, was strongly positive for anti–platelet factor 4/heparin IgG (2.855 optical density),” noted the authors, a finding that reinforces the possibility of VITT or TTS.
“Although we believe the evidence supporting VITT or TTS in this case is robust, we cannot rule out atypical HIT or HIT with unrecorded heparin administration,” concluded Kaplan and colleagues.
According to editorialists Allyson M. Pishko, MD, and Adam Cuker, MD, MS, both of the University of Pennsylvania, Philadelphia, the differences between VITT and HIT are important to note.
“Although the mechanism of VITT has only begun to be elucidated, it seems similar to but also distinct from heparin-induced thrombocytopenia (HIT) in certain respects. In both VITT and HIT, IgG antibodies bind to platelet factor 4 (PF4) on the surface of platelets, resulting in widespread platelet activation. Indeed, antibodies to complexes of PF4 and polyanion (“HIT antibodies”) have been detected in most of the confirmed cases of VITT by enzyme-linked immunosorbent assays, typically at high titers (>1.0 optical density [OD] units). However, unlike HIT, VITT occurs in the absence of antecedent heparin exposure, and VITT antibodies do not depend on the presence of heparin to bind PF4 and activate platelets,” they wrote in their accompanying editorial.
They noted the difficulties in making this diagnosis in this patient, including the confirmation of methicillin-sensitive S. aureus infection and the possibility that infection was present prior to presentation, as well as the finding of weakly positive anti-PF4 and polyanion enzyme-linked immunosorbent assay titers.
Ultimately however, Pishko and Cuker, noted that they are “… in full agreement with the authors’ decision to treat the described case as VITT and do not advocate delaying treatment in such cases. However, extra caution is needed before attributing the patient’s presentation to the mRNA-1273 vaccine.”
They concluded: “The remarkable speed with which clinicians and scientists have recognized this rare entity and developed evidence-based diagnosis and treatment guidelines should bolster public confidence in postlicensure vaccine safety monitoring. We remain confident in the safety of the SARS-CoV-2 vaccines and are encouraged by the willingness of the scientific community to report and act on suggestions of any vaccine-associated adverse events.”
-
A case study of a 65-year-old male with VITT or TTS after receiving Moderna’s mRNA vaccine illustrates the difficulties in making a diagnosis.
-
Thrombosis with thrombocytopenia cases are rare after Covid-19 vaccination, and this is the only report—to date—of VITT or TTS in an mRNA vaccine recipient.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
Kaplan and fellow researchers reported no conflicts of interest.
Pishko has received educational research grants to her institution from the Hemostasis and Thrombosis Research Society and Sanofi Genzyme.
Cukor has received research grants to his institution from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda; authorship royalties from UpToDate; and consulting fees from Synergy.
Cat ID: 125
Topic ID: 79,125,933,125,31,926,561,927,928,925,934