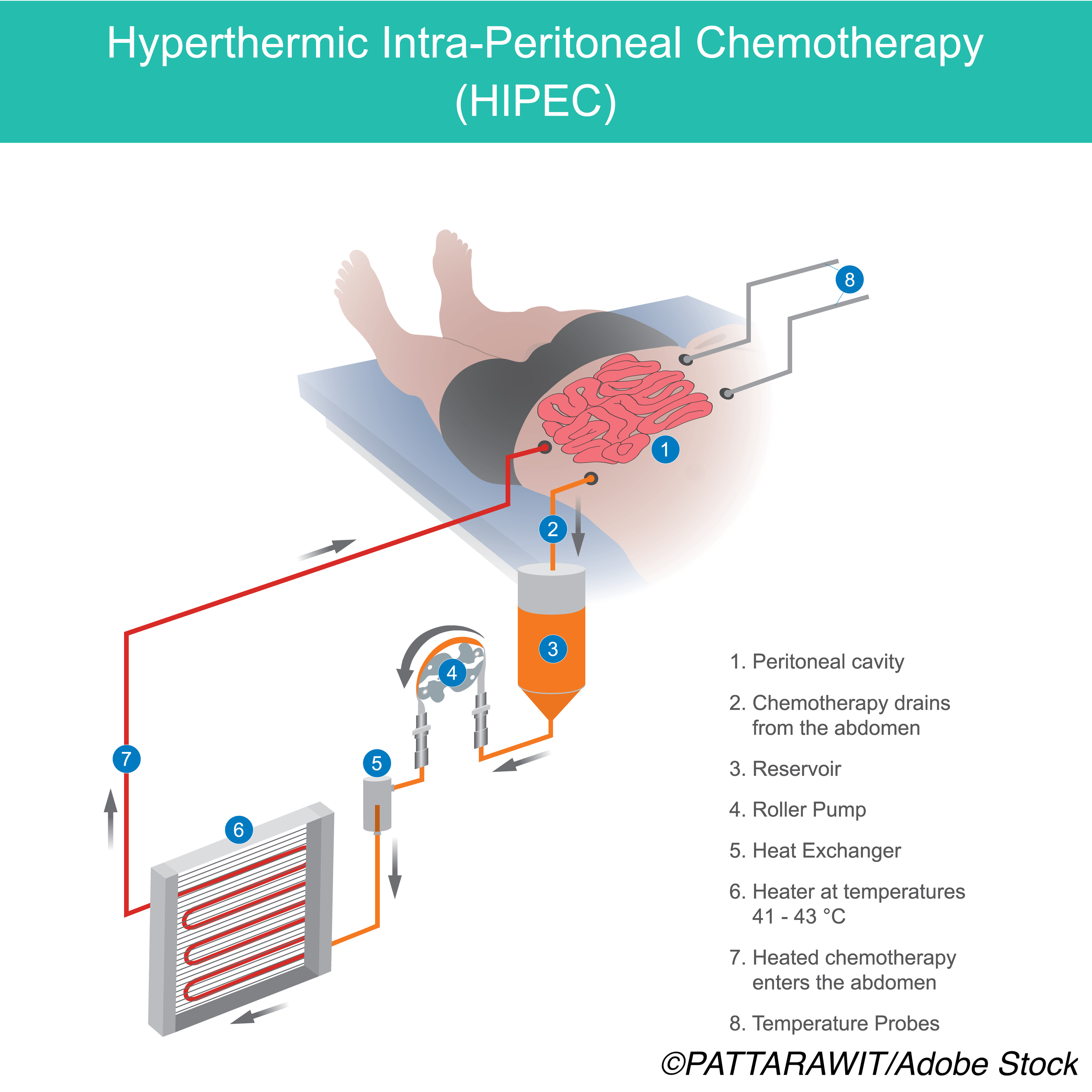
Using hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery (CRS) improved overall survival at five years compared with CRS alone in patients with pseudomyxoma peritonei (PMP), without worsening postoperative outcomes, a propensity score-matched cohort study found.
PMP is “an unusual clinical entity characterized by mucinous ascites,” most commonly originating in the appendix, which eventually lead to abdominal distension and bowel obstruction, Marcello Deraco, MD, of the Instituto Nazionale dei Milano in Italy, and colleagues explained in JAMA Surgery. And, while CRS plus HIPEC is considered the standard of care for this rare type of cancer, data on the efficacy of this treatment combination is lacking. Deraco and colleagues set out to determine the prognostic effect of HIPEC — including oxaliplatin plus fluorouracil-leucovorin, cisplatin plus mitomycin, mitomycin alone, and other oxaliplatin-based regimens — plus CRS versus CRS alone in this patient population.
In their analysis of 1,924 patients with PMP, Deraco and colleagues found that the weighted five-year overall survival was 35% longer in the CRS-HIPEC group compared with CRS alone (hazard ratio [HR] 0.65; 95% CI, 0.50-0.83; P<0.001). Specifically, 57.8% (95% CI, 50.8-65.7%) of patients in the CRS-HIPEC group were still alive at five years compared with 46.2% (95% CI, 40.3-52.8%) in the CRS alone group, investigators added. The best drug HIPEC drug schedules for increasing overall survival were oxaliplatin plus fluorouracil-leucovorin and cisplatin plus mitomycin.
The survival advantage for patients who received additional HIPEC did not come at a cost of greater postoperative complications in terms of 30- and 90-day mortality rates, severe morbidity, or the need to return to the operating room, with the exception of patients who received mitomycin alone, who experienced higher morbidity rates.
“Concern is long-standing among some surgeons using HIPEC in addition to CRS in PMP,” Deraco and colleagues wrote. “Some have suggested that HIPEC efficacy is limited owing to the poor response of PMP to chemotherapy.” However, despite this, the study authors found that “HIPEC was associated with better overall survival without worsening the postoperative outcomes in patients with PMP… [although] confirmation by a large randomized clinical trial is warranted.”
Commenting on the findings, Paul Mansfield, MD, of the MD Anderson Cancer Center in Houston, and colleagues noted that this study “seeks to provide the science to what most of us who care for patients with appendiceal tumors believe, that HIPEC helps more than it hurts.” Nevertheless, the editorialists called for caution if practitioners are to be “truly scientific.”
First, because the study extended over many years, investigators had to use an outdated 2010 World Health Organization criterion that describes tumors as only low or high grade, which the editorialists felt was limiting.
Did, for example, patients have invasive cancers in the appendix? they asked. Did the tumor deposits contain tumor cells? “These are important points, and we do not know how these factors varied between groups,” Mansfield and colleagues wrote.
The editorialists also pointed out that most HIPEC studies are “inherently confounded” because investigators use both CRS and HIPEC and then conclude that HIPEC is effective.
In the current study, fewer than 20% of patients were treated with CRS only; for these patients, “we do not know the reasons patients did not receive HIPEC,” the editorialists observed, and these reasons could make a difference to outcomes, as tumors treated with CRS alone could have been more invasive or the patients less stable.
The editorialists also noted that in trying to determine which HIPEC regimen offers the best outcomes, the purportedly “best” regimen was oxaliplatin plus fluorouracil/leucovorin. However, at five years, this particular arm contained only two evaluable patients compared with 47 patients in the CRS-only arm.
This discrepancy in patient numbers raises questions about whether patients in the most favorable HIPEC arm simply received treatment in the most recent period, when oxaliplatin-based regimens had become more popular but so, too, have improvements in perioperative management, patient selection, and other therapies.
“This study may provide some insights but randomized clinical trials will remain the mainstay for moving us forward,” Mansfield and colleagues concluded.
For their analysis, the study authors pulled data on 1,924 patients with PMP (51.8% men; median age, 56) from the Peritoneal Surface Oncology Group International (PSOGI) registry — of these, 376 patients underwent CRS alone while 1,548 patients were treated with CRS followed by HIPEC.
“The surgical approach involved peritonectomy procedures… [and] HIPEC was administered after CRS using an open coliseum or closed technique,” the authors explained.
The most frequent HIPEC drug regimens used included mitomycin, given as either a fixed dose of 40 mg or at a dose of 35 mg/m22 plus the combination of fluorouracil and leucovorin 400 mg/m222 per liter of perfusate associated with mitomycin 3.3 mg/m2 per liter of perfusate. The perfusate was heated to achieve a temperature ranging from 40 to 43° C, they added.
During a median follow-up of 52 months (interquartile range (IQR), 23-83 months), the HIPEC drug schedule associated with the best overall survival was oxaliplatin plus fluorouracil/leucovorin at 86.5% (95% CI, 0.76-0.97%). The overall survival rate at five years for CRS alone was only 62.6% (95% CI, 0.54-0.73%; P=0.03).
Second best was the HIPEC doublet consisting of cisplatin plus mitomycin, where overall survival at five years was 64.9% (95% CI, 58.0-72.7%) compared with 52.2% (95% CI, 45.8-59.4%; P<0.001) for CRS alone, they added.
In contrast, “[n]o prognostic advantage was observed in subgroups receiving mitomycin,” the authors noted, where five-year overall survival rates were 58.8% (95% CI, 50.1-69.0%) in the CRS-HIPEC group compared with 59.1% (95% CI,51.9-67.2%; P=0.68) for the CRS. Mitomycin alone was also associated with an increased risk of morbidity (odds ratio 1.99; 95% CI, 1.25-3.19; P=0.004).
Thirty-day mortality rates were 62% lower in those who received additional HIPEC, while 90-day mortality rates were 53% lower compared with CRS-alone-treated patients. Similarly, the need to return to the operating room was 36% lower for HIPEC-treated patients compared with those treated with CRS alone, although morbidity rates were similar between the two groups.
CRS-HIPEC treated patients also had better five-year overall survival compared with CRS alone in all subsets of patients including those who had optimal cytoreduction—defined as residual disease of less than 2.5 mm—as well as those who had suboptimal cytoreduction—defined as residual disease in excess of 2.5 mm. Patients with both low- and high-grade disease also fared considerably better when they received additional HIPEC compared with CRS alone, the authors pointed out.
-
Cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) improved OS at five years in PMP patients, especially those treated with a regimen containing oxaliplatin plus fluorouracil-leucovorin.
-
Treating PMP patients with additional HIPEC was not accompanied by worsening postoperative outcomes for most patients with the exception of those who received HIPEC in the form of mitomycin alone.
Pam Harrison, Contributing Writer, BreakingMED™
Coauthor Glehen reported receiving personal fees from Gamida Cell, Ltd, during the conduct of the study. Coauthor de Hingh reported receiving grants from F. Hoffman–La Roche Ltd, Kankerbestrijding, and Quality in Products and Services/RanD outside the submitted work.
The editorialists had no disclosures to report.
Cat ID: 935
Topic ID: 78,935,730,188,935,192,925,159


