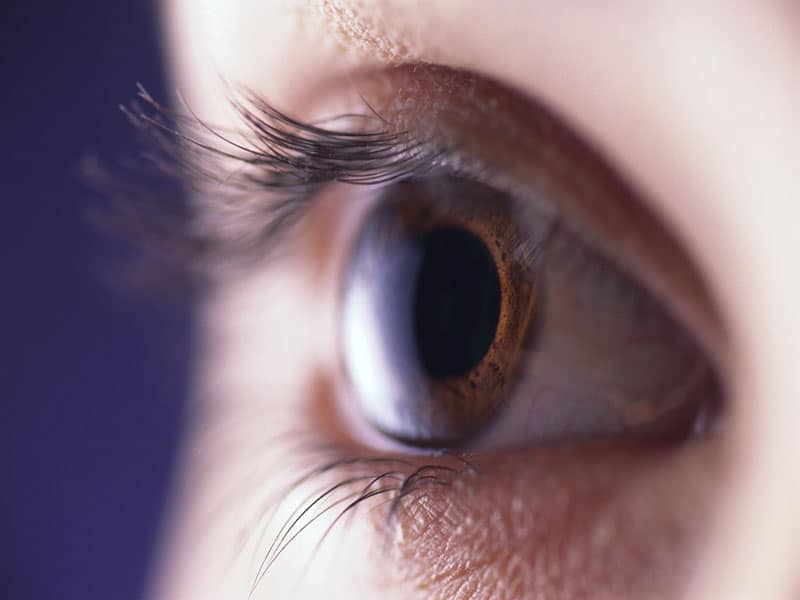
THURSDAY, May 31, 2018 (HealthDay News) — The first artificial iris has been approved by the U.S. Food and Drug Administration for patients with aniridia.
The CustomFlex Artificial Iris is custom-colored and molded for each user from medical-grade silicone. The device was evaluated in clinical trials involving almost 400 adults and children. More than 70 percent of users reported decreased light sensitivity and glare, and 94 percent of users were satisfied with the device’s appearance, the FDA said.
The most common side effects included device movement or dislocation, increased intraocular pressure, iritis, synechiae, and the need for additional surgery to reposition, remove, or replace the device. The device isn’t recommended for pregnant women or for people with certain medical conditions affecting the eye, the agency said.
“Patients with iris defects may experience severe vision problems, as well as dissatisfaction with the appearance of their eye,” Malvina Eydelman, M.D., director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s approval of the first artificial iris provides a novel method to treat iris defects that reduces sensitivity to bright light and glare. It also improves the cosmetic appearance of the eye in patients with aniridia.”
The CustomFlex Artificial Iris is produced by the German firm HumanOptics AG.
Copyright © 2018 HealthDay. All rights reserved.