After cardiac surgery, continuous cardiac rhythm monitoring in patients with a high risk of stroke, no history of preoperative atrial fibrillation (AFib), and AFib of <24 hours during hospitalization detected a significant increase in the rate of postoperative atrial fibrillation (POAF) after discharge that would otherwise have gone unnoticed with usual care.
Researchers urged further studies to assess whether oral anticoagulative treatments would be beneficial in such patients. Their results are published in JAMA Network Open.
Subodh Verma, MD, PhD, of University of Toronto, Ontario, Canada, and the SEARCH AF CardioLink-1 Investigators sought to assess whether continuous cardiac rhythm monitoring improves the detection of POAF in cardiac surgical patients for the first 30 days after hospital discharge compared with usual care.
“POAF after cardiac surgery occurs in 30% to 50% of patients during their hospital stay, with the incidence peaking at 3 to 5 days and decreasing afterwards. However, the incidence of POAF after discharge from cardiac surgery is not well defined. Most studies have been limited to the hospitalization phase only and were small, nonrandomized, or included patients with antecedent AFib and used limited monitoring for AFib. Among cardiac surgical patients who experienced little to no POAF during hospitalization, their risk of experiencing AFib after discharge is unknown,” they wrote.
For this open-label, multicenter, randomized clinical trial, they enrolled 336 cardiac surgical patients (mean age: 67.4 years; 21.7% women; median CHA2DS2-VASc score: 4.0) with CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years, diabetes, prior stroke or transient ischemic attack, vascular disease, age 65-74 years, female sex) ≥4 or ≥2 with risk factors for POAF, no history of preop AFib, and POAF lasting ˂24 hours during hospitalization.
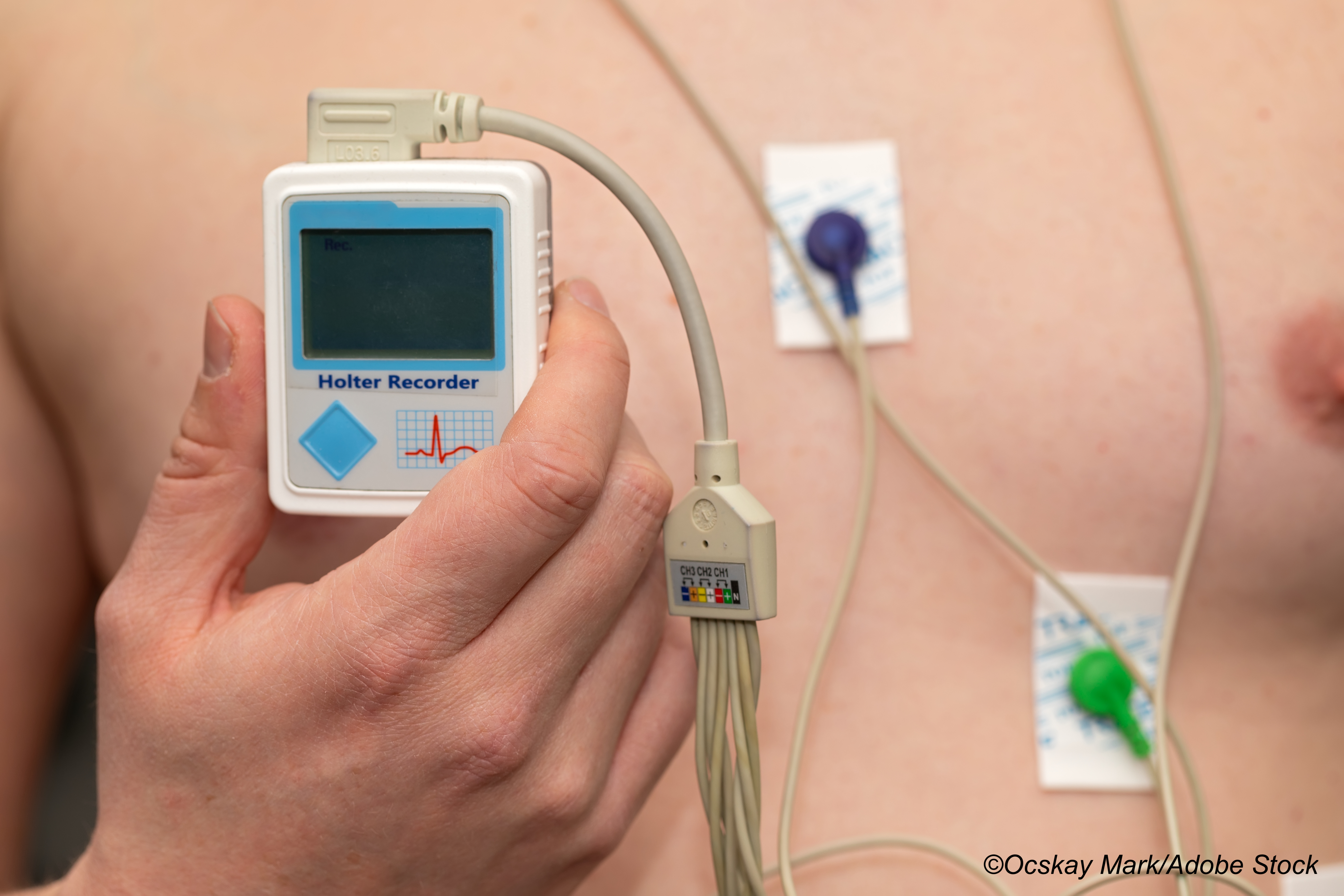
Patients were randomized to continuous cardiac rhythm monitoring with a wearable, patch-based monitor for 30 days or usual care. Continuous ECG monitoring was done with a wearable, adhesive patch monitor (Medtronic’s SEEQ system and Incentia’s CardioSTAT system). Patients were instructed on correct monitoring, and then contacted in weekly or biweekly follow-ups. In all, 307 completed the trial.
Upon intent-to-treat analysis, the primary endpoint of cumulative AFib and/or atrial flutter lasting 6 minutes or longer occurred in 19.6% of patients who underwent continuous cardiac rhythm monitoring, compared with 1.7% of patients in the usual care group (absolute difference: 17.9%; 95% CI: 11.5%-24.3%; P<0.001), and AFib duration of ≥6 hours was detected in 8.6% versus 0, respectively (absolute difference: 8.6%; 95% CI: 4.3%-12.9%; P<0.001).
First occurrences of AFib or atrial flutter of 6 minutes or longer was detected in 73.3% of patients during week 1 of monitoring, in 20.0% during week 2, and in 6.7% during week 3.
In the 45 days after discharge, 3.3% of patients were given oral anticoagulation therapy (4.3% from the cardiac monitoring group and 2.3% of those on usual care), and from 46 post-discharge to the end of follow-up, 3.7% and 2.9%, respectively, were given anticoagulative therapy.
Jim Cheung, MD, chair-elect of the American College of Cardiology Electrophysiology Council, reviewed the study for BreakingMED.
“This study shows that among patients undergoing cardiac surgery who have no prior known history of atrial fibrillation (AFib), post-discharge continuous cardiac rhythm monitoring can detect AFib in almost 20% of patients. The majority of these detections occur during the first 2 weeks after discharge and would have otherwise been missed with usual care. Since most patients undergoing cardiac surgery are at elevated risks of stroke if they have AFib, this has implications for whether or not extended post-surgical cardiac rhythm monitoring should be considered to guide AFib detection and treatment with anticoagulation for stroke prevention if needed. The strength of this study lies in its randomized design and excellent follow-up,” he said.
He noted that currently, no guidelines call for routine post-discharge cardiac rhythm monitoring for AFib in patients undergoing cardiac surgery with no signs of AFib during index hospitalization.
He explained: “The vast majority of patients would undergo routine discharge follow-up in the office and receive a 12 lead ECG. If the patients had brief (e.g., <24 hours) AFib detected during hospitalization, I believe that usual care may vary among clinicians. In my practice, the detection of even relatively limited duration of episodes of AFib, such as < 24 hours as defined by this study, would trigger initiation of anticoagulation. Outpatient follow-up with extended cardiac rhythm monitors would be performed at 3-month post-surgery to assess whether or not anticoagulation should be continued. There are still many unanswered questions such as: How much AFib is enough to trigger anticoagulation? What are the parameters to define when it is safe to stop anticoagulation—is it 12-lead ECG, 2-week monitoring or insertable loop recorder?”
Are these results surprising? According to Cheung, they are not.
“These results are not surprising and are in line with mounting evidence that the harder you look for AFib, the more you find, particularly in patients who are at elevated baseline risk for the arrhythmia. Specifically, up to 44% of newly detected post-operative AFib can be found in patients after cardiac surgery during hospitalization. Although this is often thought to be transient, there is little doubt that risks of recurrent AFib in this high-risk patient population is significant, as underscored by this study. This is consistent with findings from studies of patients with AFib after non-cardiac surgery that show that a significant proportion of patients with so-called ’postoperative AFib’ will in fact have recurrent AFib over long-term follow-up,” he told BreakingMED.
Should they change current clinical practice?
“These results should clearly raise our awareness of the significant prevalence of subclinical AFib detected in the post-discharge setting after hospitalization for cardiac surgery. Unfortunately, it was beyond the scope of this study to explore the impact of AFib detection in this setting on guiding anticoagulation to reduce important endpoints such as stroke or mortality. Therefore, it remains to be seen whether or not routine extended cardiac rhythm monitoring should be adopted in all patients at elevated risk for AFib after discharge from cardiac surgery as a way to guide anticoagulation management. A future study with insertable loop recorders that can provide several years of monitoring in this patient population may be able to better answer this question,” Cheung concluded.
Study limitations include the 30-day primary end point measurement, failure of patients to complete limited continuous monitoring, exclusion of patients with AFib lasting more than 34 hours during hospitalization and those with a prolonged hospital stay, failure to perform preoperative cardiac monitoring to exclude preoperative AFib, and some patients’ lack of adherence to monitoring.
-
In cardiac surgical patients with risk factors for stroke and atrial fibrillation (AFib) lasting less than 24 hours postoperatively, continuous cardiac rhythm monitoring significantly improved detection of AFib during the first 30 days after hospital discharge compared with usual care.
-
Researchers from the SEARCH AF CardioLink-1 study urged further studies to determine if these patients would benefit from oral anticoagulation therapy.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
This study was funded by a grant from the Heart and Stroke Foundation of Canada, Canadian Stroke Prevention Intervention Network, and unrestricted investigator-initiated grants from Bristol-Myers Squibb-Pfizer Alliance and Boehringer Ingelheim. Medtronic provided in-kind support with SEEQ devices.
Verma reported holding a Tier 1 Canada Research Chair in Cardiovascular Surgery; reported receiving research grants and speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm, Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group; and reported being the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization.
Cat ID: 2
Topic ID: 74,2,730,2,8,913,914,38,192,925