Lynne Peterson is the Senior Writer for Trends-in-Medicine.
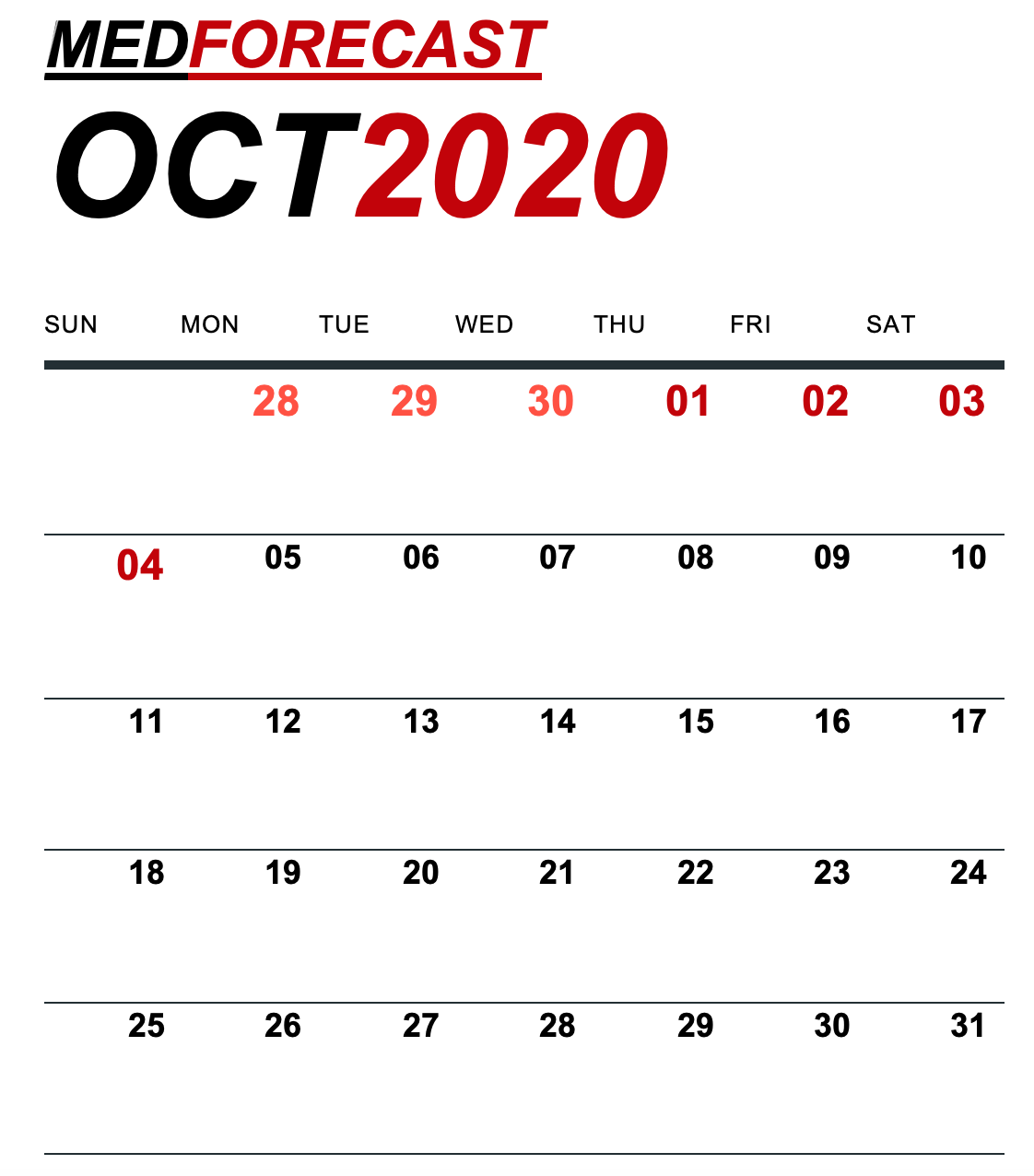
Cardiology: The Heart Failure Society of America (HFSA) virtual annual scientific meeting will take place Sept. 30-Oct. 6 (yes, 7 days of which 2 are full days and 5 are evening sessions). There will be the debates, science, late-breaking clinical trials, and discussion sessions you have come to expect from the in-person HFSA conferences. Among the trial data to be presented is the failed pPhase II study of Tenax Therapeutics’ levosimendan in pulmonary hypertension associated with heart failure and preserved ejection fraction (PH-HFpEF).
Endocrinology: The FDA is expected to make a decision by Sept. 29 on a treatment for pediatric adrenal insufficiency – Eton Pharmaceuticals and Diurnal’s Alkindi Sprinkle.
Neurology
- The World Muscle Society (WMS) virtual meeting will take place Sept. 28-Oct. 2. Registration is “full,” but you can get on the waiting list. The topics include new developments in congenital muscle disease, gene modifiers and gene delivery in neuromuscular disorders, and advances in the treatment of neuromuscular disorders.
- The American Neurological Association (ANA) virtual meeting will start Oct. 3 with a special Social Justice Symposium and run through Oct. 9. The focus will be on the latest advances in translational neuroscience, neurobiology of disease, academic neurology, and advances in the various neurologic subspecialties.
Oncology
- The European Breast Cancer Conference (EBCC) will take place virtually October 2-3. Topics include surgery, state-of-the-art radiotherapy and imaging, strategies for treating metastatic breast cancer, how to reduce overtreatment, late-breaking trials, immuno-oncology, and much more.
- The North American Neuroendocrine Tumor Society (NANETS) Multidisciplinary NET Medical Virtual Symposium will take place October 2-3. Learn more about assessing response in NETS, sequencing care in advanced NETS, innovative approaches to NET care delivery, cutting edge NET research, and more.
- The Immunotherapy of Cancer virtual conference (ITOC7) will take place Oct. 2-3. Look for phase Ia/Ib dose-escalation data on a STING agonist in solid tumors – Spring Bank Pharmaceuticals’ SB-11285.
Ophthalmology: The EURETINA 2020 virtual meeting will take place October 2-4. Among the data to be presented are the results of:
- A phase I/II trial of a subretinal gene therapy – MeiraGTx’s AAV-RPGR – for X-linked retinitis pigmentosa.
- A phase III trial of a Complement C3 inhibitor – Apellis Pharmaceuticals’ pegcetacoplan – in geographic atrophy (GA) associated with dry age-related macular degeneration (AMD).
Regulatory
- The FDA will host a virtual public meeting on Sept. 29 on patient preference information use in medical device regulatory decisions.
- The FDA will hold a two-day virtual public workshop on Sept. 29 and Oct. 1 on FDA regulation of reproductive tissues. (Note that there is a gap between the two days of the workshop.)
- The FDA’s National Center for Toxicological Research will host a virtual global summit on September 30 on regulatory science, focused on emerging technologies.
- The FDA is holding a virtual public meeting on Sept. 30 on patient-reported outcomes (PROs) and medical device evaluations.
- The FDA is holding a webinar on Oct. 2 on controlling nitrosamine impurities in human drugs.
- The FDA’s Vaccines and Related Biological Products Advisory Committee will meet virtually on Oct. 2 to discuss the strains to be included in the flu vaccine for the 2021 southern hemisphere flu season (that’s South America, not the U.S.).
Respiratory: The FDA is expected to make a decision by Sept. 30 on a treatment for steroid-refractory acute graft-versus-host disease (GVHD) in pediatric patients – Mesoblast’s Ryoncil (remestemcel-L).
Vaccines: The World Vaccine Congress will take place virtually Sept. 28-Oct. 1. Among the topics will be: influenza and respiratory disease, cancer and immunology, clinical trials, how government agencies are responding to vaccine challenges, vaccine hesitancy, and the future of the vaccine landscape. Among the speakers will be Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert Johnson, PhD, director of the Division of Influenza and Emerging Infectious Diseases at the Biomedical Advanced Research and Development Authority (BARDA), as well as officials from several major pharmaceutical companies involved in vaccine research.
Lynne Peterson, Contributing Writer, Senior Writer for Trends-in-Medicine
Cat ID: 707
Topic ID: 74,707,730,707,187,935,31,130,208,192,561,418,725,195,925,240