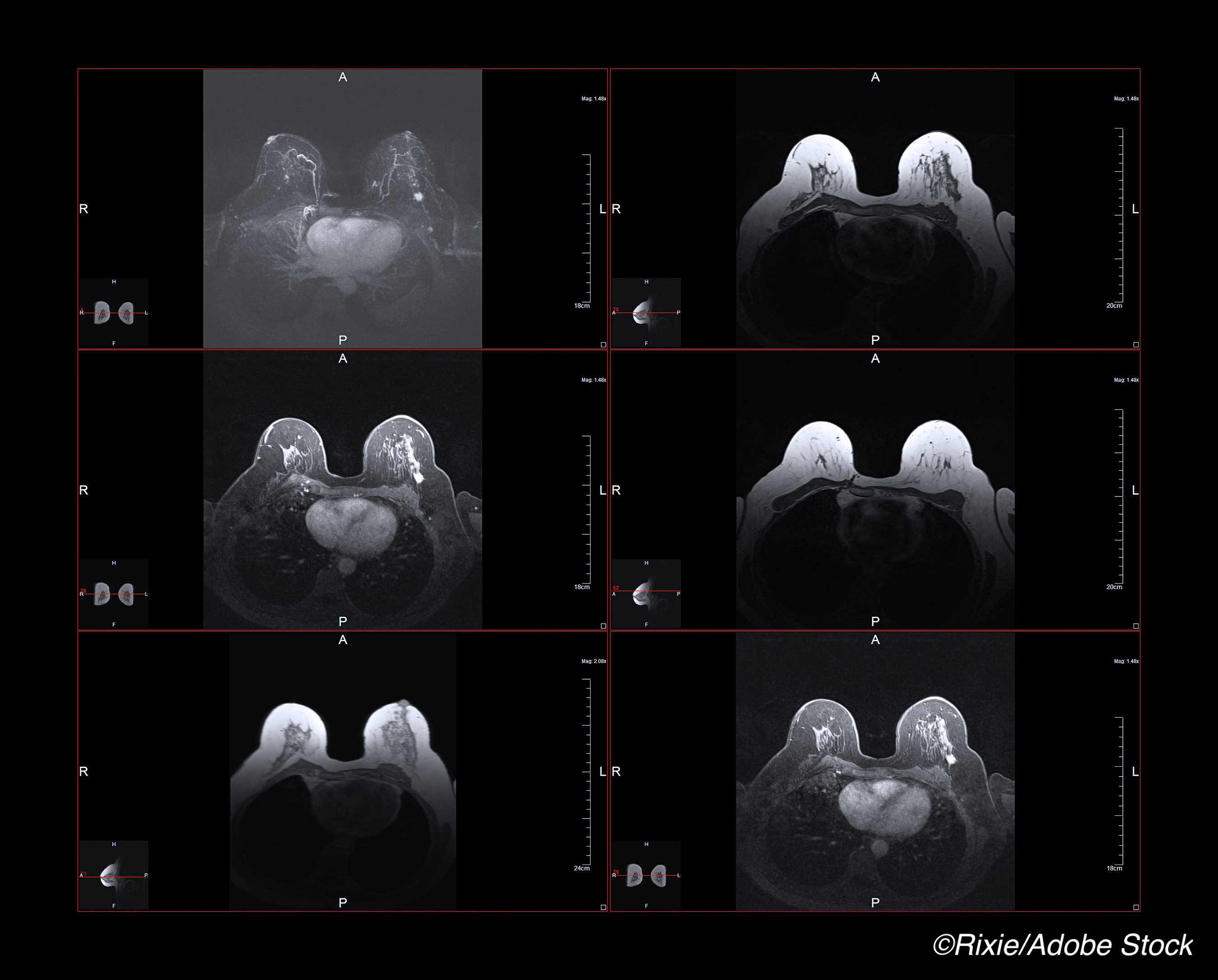
Magnetic resonance imaging (MRI)-guided biopsy was almost as accurate as reference-standard surgical resection in its ability to diagnose a pathological complete response (pCR) following neoadjuvant chemotherapy for breast cancer, a small pilot study found.
In a cohort of 20 evaluable patients, MRI-guided biopsy had a negative predictive value (NPV) of 92.8% (95% CI 66.2%-99.8%)—the primary end point of the study—when pCR was defined as no residual invasive disease, as well as an NPV of 85.8% (95% CI, 57.2%-98.1%) when pCR was defined as no residual invasive or in situ cancer, Elizabeth Sutton, MD, of the Department of Radiology at Memorial Sloan Kettering Cancer Center in New York City, and colleagues reported in JAMA Network Open.
MRI-guided biopsy also had a positive predictive value (PPV) of 100%, an accuracy of 95% (95% CI, 75.1%-99.9%), and a specificity of 100% when pCR was defined as no residual invasive cancer, investigators noted.
“After neoadjuvant chemotherapy [neoadjuvant chemotherapy], pathologic complete response (pCR) is an optimal outcome and a surrogate end point for improved disease-free and overall survival,” Sutton and colleagues observed.
Based on these results, the study authors concluded that “MRI-guided biopsy may be a viable alternative to surgical resection for this population after [neoadjuvant chemotherapy], which supports further investigation.”
The single-arm, phase I, nonrandomized controlled trial was carried out in a single tertiary care cancer center between September 2017 and July 2019. Patients recruited to the trial had stage IA to IIIC biopsy-proven operable invasive breast cancer, and 95% (n=19) had invasive ductal carcinoma. Some 75% had stage II cancer; 55% had ERBB-2 positive cancer, and 30% had triple-negative breast cancer.
Findings from surgical pathology found that 35% of patients did not achieve a pCR following neoadjuvant chemotherapy while 65% did when pCR was again defined as no residual invasive disease.
“All MRI-guided biopsies were performed with a 1.5 or 3.0-T whole-body MRI unit (GE Discovery 450w or 750; GE Medical Systems),” Sutton and colleagues noted. Standard-of-care MRI-guided biopsy was done in all patients, although no intravenous gadolinium was used in this particular study.
For surgical comparisons, 7 to 12 biopsy samples were harvested and sent to pathology for analysis. “Of the 20 patients, the pre-[neoadjuvant chemotherapy] median tumor size on MRI was 3.0 cm (interquartile range (IQR), 2.0-5.0 cm),” investigators noted.
At a median follow-up of 1.26 years (IQR, 0.85-1.59 years), surgical pathology results identified residual invasive cancer in 40% of the group, ductal carcinoma in situ but no invasive cancer in 5% of patients, and no residual invasive or in situ cancer in 55% of the group overall.
When a pCR was defined as no residual invasive cancer, there was one false-negative MRI-guided biopsy result where surgical pathological findings, in contrast, found less than 0.02 cm of residual invasive disease.
When a pCR was defined as no residual invasive or in situ cancer, there was a second false-negative MRI-guided biopsy result for which findings on surgical pathology showed residual ductal carcinoma in situ.
Neither age nor breast cancer subtype were associated with surgical pathology findings using either definition of pCR, the study authors noted. On the other hand, MRI-guided biopsy findings were positively associated with surgical pathology, again using both definitions of pCR.
As the authors pointed out, the two patients whose MRI-guided biopsy results were falsely negative based on a definition of pCR as having no residual invasive or in situ cancer, had a focus of either ductal carcinoma in situ or a microscopic focus of residual invasive cancer.
“Hence, these findings suggest that a negative MRI-guided biopsy result was a reliable indicator of pCR, or limited residual disease, after [neoadjuvant chemotherapy],” Sutton and colleagues observed.
“[And t]his image-based approach could potentially represent a minimally invasive alternative to a surgical procedure of unclear benefit,” they added.
The authors cautioned that their analyses are limited by the small sample size and that their methodology was dependent on advanced breast MRI imaging approaches, and therefore extrapolation of their findings to other imaging modalities must be made with caution.
-
MRI-guided biopsy was almost as accurate as surgical resection in its ability to diagnose a pathological complete response following neoadjuvant chemotherapy for breast cancer.
-
MRI-guided biopsy may prove to be a minimally invasive alternative to surgical resection and pathology to determine response to initial treatment for breast cancer patients.
Pam Harrison, Contributing Writer, BreakingMED™
Coauthor Morris reported receiving funding from the Susan G. Komen Foundation and Grail Inc. for breast cancer research not related to the contents of this article. No other disclosures were reported.
Cat ID: 22
Topic ID: 78,22,730,22,691,192,925


