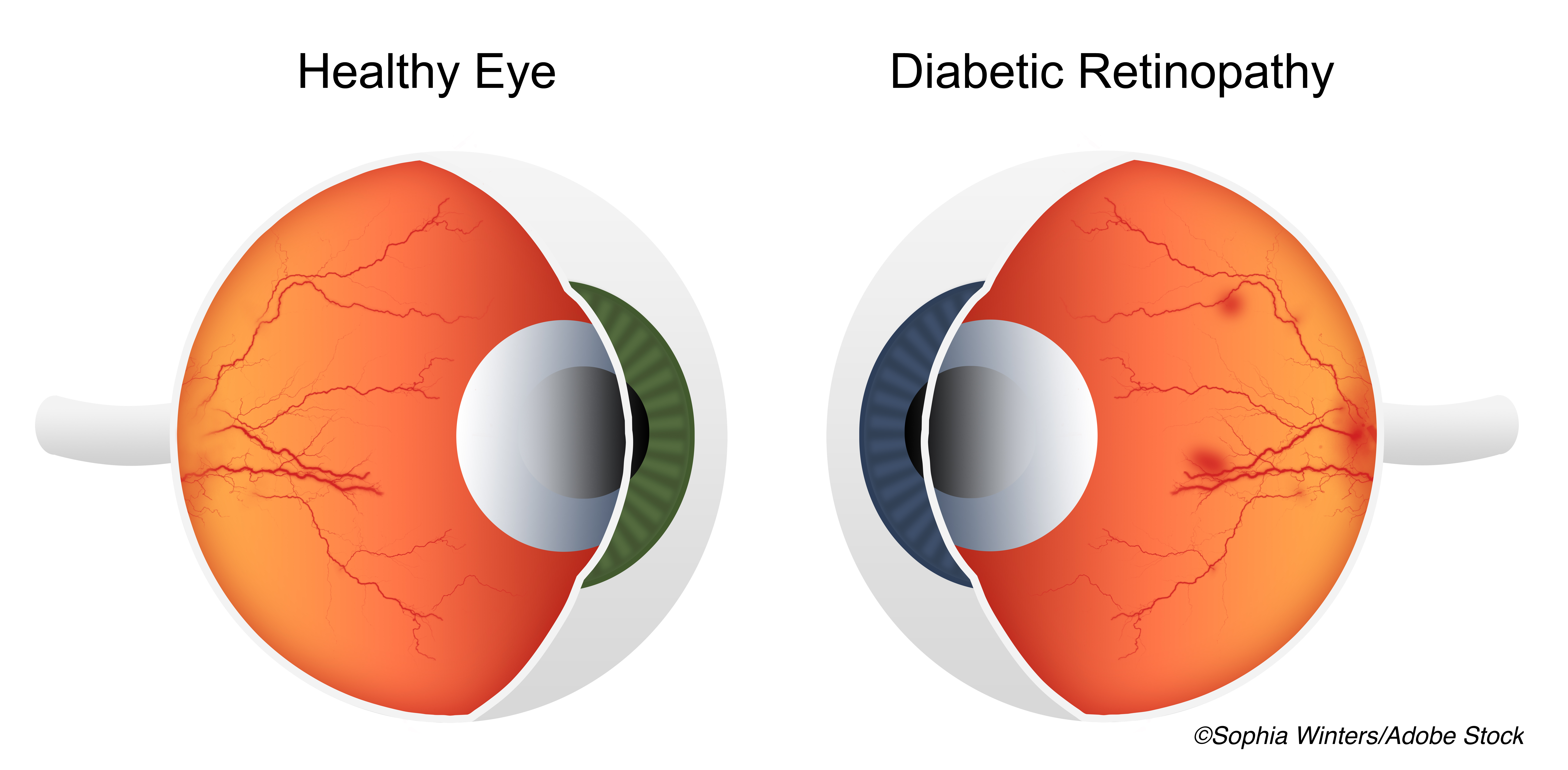
In patients with moderate-to-severe non-proliferative diabetic retinopathy (NPDR), aflibercept injections reduced vision-threating complications, with greater than a three-fold reduction in the incidence of center-involved diabetic macular edema (CI-DME) and more than a two-fold reduction in the incidence of proliferative diabetic retinopathy (PDR).
However, over two years, treatment with aflibercept brought about no improvements in visual acuity compared with a course of observation plus aflibercept (if complications developed), according to a study conducted by researchers from the DRCR Retina Network who published the findings in JAMA Ophthalmology.
“The DRCR Retina Network Protocol W was designed as a long-term study to determine whether there is a benefit of aflibercept for a 2- and 4-year period for the prevention of PDR or CI-DME in eyes with moderate to severe NPDR, and if so, whether there is an associated visual benefit of aflibercept for the prevention of PDR or CI-DME with vision loss compared with observation and aflibercept treatment if vision-threatening complications develop,” wrote Raj K. Maturi, MD, of the Indiana University school of Medicine’s Midwest Eye Institute, Indianapolis, and colleagues.
They enrolled 328 adults (median age: 57 years; 57.6% men; 46.7% White; 30.5% Hispanic or Latino; 15.2% African American) with NPDR without CI-DME, randomizing 399 eyes to treatment with either intravitreal aflibercept (2 mg) or sham injections, which were given at baseline, one, two, and four months, and then every four months over two years.
Patients treated with aflibercept had a 2-year cumulative probability of CI-DME with vision or NPDR of 16.3%, compared to 43.5% in those treated with the sham (overall HR for either: 0.32; 97.5% CI: 0.21-0.50; P˂0.001 in favor of aflibercept).
But while this rate was three-fold less than that in the sham group, Maturi et al stressed: “This finding demonstrates that anti-VEGF treatment as provided in this study does not guarantee prevention of vision-threatening complications in this high-risk cohort. Continued ophthalmic follow-up and routine examinations are needed to diagnose and treat PDR and CI-DME irrespective of whether intravitreal anti-VEGF therapy is given for prevention of these conditions.”
Similarly, the two-year cumulative probability of developing PDR was significantly lower in patients treated with aflibercept compared with sham injections (13.5% vs 33.2%, respectively), as was the two-year cumulative probability of developing CI-DME with vision loss (4.1% vs 14.8%).
Finally, changes in visual acuity between the two groups from baseline were not significantly different, with a mean change from baseline to two years of −0.9 letters in patients treated with aflibercept compared with −2.0 letters in patients treated with sham (adjusted mean difference: 0.5 letters; 97.5% CI: −1.0 to 1.9 letters; P=0.47).
A pair of commentaries accompanied the study, one by Jennifer I. Lim, of the University of Illinois at Chicago, who is an associate Deputy Editor of JAMA Ophthalmology, and the other by Rajendra S. Apte, MD, PhD, and Christopher K. Hwang, MD, PhD, both of the Washington University in St. Louis School of Medicine.
Linn wrote that the prophylactic treatment with aflibercept injections in this patient population to prevent severe NPDR is “reasonable based on a careful evaluation of risks and benefits of therapy.” But more is needed, thus the ongoing four year follow-up “will be very helpful in deciding whether to implement this treatment since Protocol W institutes an as-needed regimen after year 2. Durability of outcome on DRSS regression and as-needed use of aflibercept for prevention of CI-DME and PDR at 4 years would support prophylactic treatment.”
Apte and Hwang echoed Linn’s call for caution: “Despite substantial differences in the 2 primary end points, which were anatomic outcomes, prophylactic aflibercept for eyes with moderate or severe NPDR and good vision may not be cost-effective or practical in the clinical practice setting,” wrote Apte and Hwang. They pointed out that the follow-up is scheduled for completion in 2022, thus waiting “…no more than 2 years from now for 4-year visual acuity outcomes may be warranted for eyes with moderate to severe NPDR, as these eyes are monitored safely in Protocol W. The 4-year follow-up could also shed some light on the natural history of aflibercept-treated eyes with mild NPDR (as these eyes would not receive aflibercept in years 2 to 4 per study protocol) and provide some reference for comparison with anti-VEGF naïve eyes with mild NPDR,” they concluded.
Study limitations include bias due to loss of patients to follow-up, unmasking of investigator charged with assessing components used to determine the primary outcome, possibility that some outcome components such as vitreous hemorrhage may not be cause by diabetic pathologic conditions, and the non-generalizability of the treatment algorithm with aflibercept to other anti-VEGF agents or treatments.
-
Aflibercept injections reduced the development of vision-threating complications; however, through two years, preventive treatment did not confer visual acuity benefit compared with observation plus aflibercept if complications developed.
-
In patients with moderate-to-severe nonproliferative diabetic retinopathy, the two-year rate of developing center-involved diabetic macular edema with vision loss or proliferative diabetic retinopathy was 16.3% with aflibercept versus 43.5% with sham, but between-group differences in twp-year mean visual acuity change was only 0.5 letters.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
The study was supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, US Department of Health and Human Services EY14231. Regeneron provided aflibercept for the study and funds to DRCR Retina Network to defray the study’s clinical site costs. Additional grants to the Jaeb Center for Health Research from the JDRF. Network chairs, coordinating center staff, committee members, and the reading center staff are all compensated for their work as members of the DRCR Retina Network.
Maturi reported receiving personal fees from Jaeb Center for Health Research during the conduct of the study; personal fees from Aerpio, Allegro, Allergan, Eli Lilly, Genentech, Graybug, Kalvista, and Santen outside the submitted work.
Apte received support from the Jeffrey Fort Innovation Fund, the Starr Foundation, and Research to Prevent Blindness, Inc.
Hwang reported no disclosures.
Cat ID: 240
Topic ID: 92,240,730,12,192,669,918,240