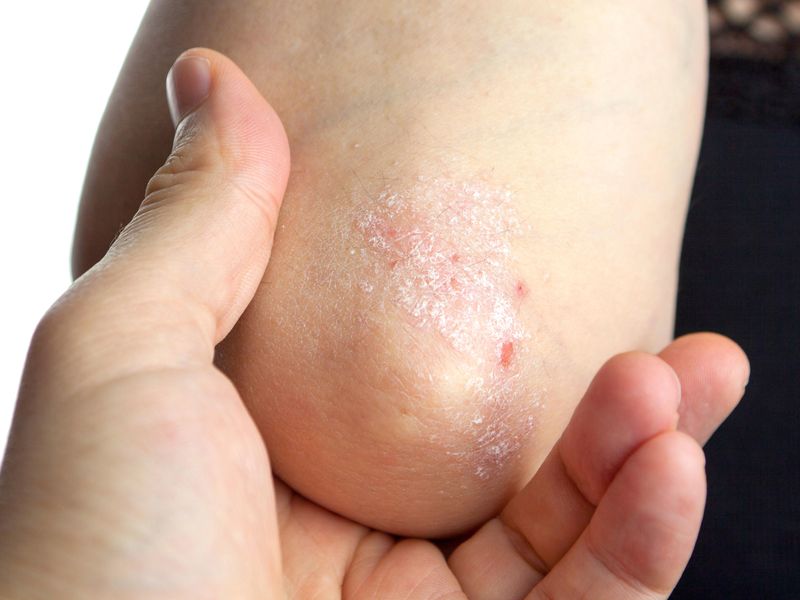
WEDNESDAY, July 13, 2022 (HealthDay News) — Four in 10 plaque psoriasis patients achieve complete disease clearance with tapinarof cream, 1 percent, according to a study published online June 26 in the Journal of the American Academy of Dermatology.
Bruce Strober, M.D., Ph.D., from Yale University in New Haven, Connecticut, and colleagues assessed the long-term safety, efficacy, remittive effect, durability of response, and tolerability of tapinarof for the treatment of mild-to-severe plaque psoriasis. The analysis included 763 patients who completed the 12-week trial and were eligible for 40 weeks of open-label treatment and four weeks of follow-up.
The researchers found that 40.9 percent of patients achieved complete disease clearance (Physician Global Assessment [PGA] of 0). More than half of participants (58.2 percent) entering with PGA ≥2 achieved PGA of 0 or 1. For patients achieving PGA of 0, mean duration of remittive effect off-therapy was 130.1 days. No new safety signals were seen. Folliculitis (22.7 percent), contact dermatitis (5.5 percent), and upper respiratory tract infection (4.7 percent) were the most frequent adverse events.
“VTAMA [tapinarof] cream’s demonstrated combination of efficacy, remittive effect, durability, and tolerability make it a strong addition to the psoriasis armamentarium,” Philip M. Brown, M.D., J.D., chief medical officer of Dermavant Sciences, said in a statement.
Several authors disclosed financial ties to pharmaceutical companies, including Dermavant, which manufactures tapinarof and funded the study.
Abstract/Full Text (subscription or payment may be required)
Copyright © 2022 HealthDay. All rights reserved.