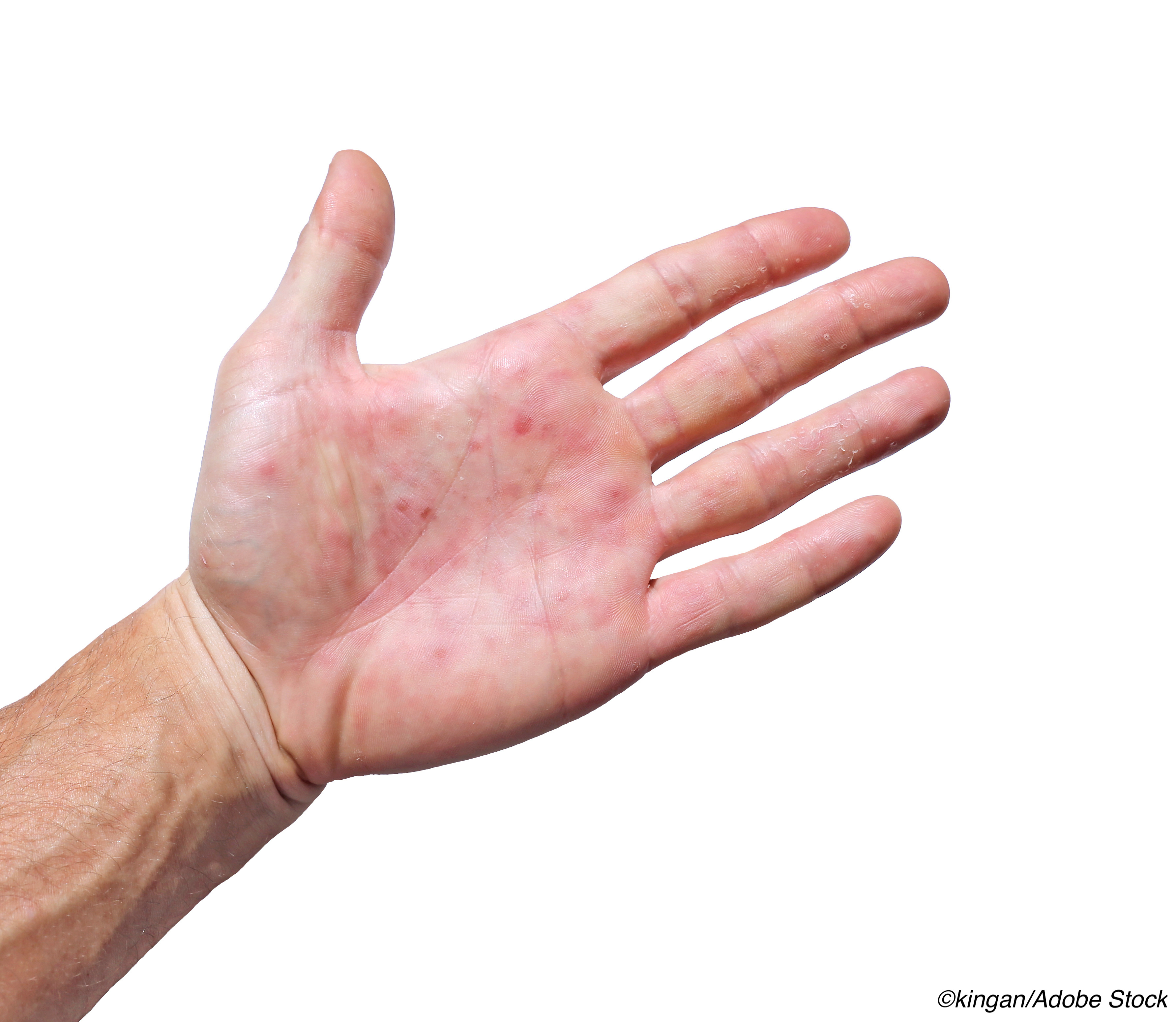
Targeting immunologic pathways may be the fast-lane treatment to clinically significant improvement in patients with chronic erythema multiforme (CEM), researchers reported.
In a 35-patient retrospective cohort study, treatment with continuous thalidomide was tied to a complete remission (CR) in 66% at six months, and a decrease in yearly flares in those without CR, according to Laurence Le Cleach, MD, PhD, from Henri Mondor Hospital in Créteil, France, and co-authors.
The authors reported in JAMA Dermatology that 71% of patients stopped thalidomide treatment after a median of 12 months, mostly because of neuropathy or another adverse effects (AEs, 56%).
“The findings of this cohort study suggest that thalidomide may be a useful alternative therapy for chronic EM,” Le Cleach’s group wrote, although they noted that because of long-term AEs, “the search for a minimal maintenance dose is essential in patients with good response.”
A separate brief report in JAMA Dermatology showed that treatment with Janus kinase (JAK) inhibitors also brought about good clinical results, but without AEs. While the case series was very small (n=4; all female; mean age 46.2; all with persistent EM), treatment with tofacitinib (Xeljanz) or upadacitinib (Rinvoq) offered “marked clinical improvement with clear or nearly clear skin,” according to William Damsky, MD, PhD, of the Yale School of Medicine in New Haven, Connecticut, and co-authors.
They pointed out that one of the four patients was previously treated with thalidomide but had no response, while another had a partial response. “These study findings suggest that while TNF [tumor necrosis factor] activity may play a role in [persistent] EM pathogenesis, it is not a proximal driver of the immune response,” Damsky’s group wrote, adding that the effectiveness of JAK-based therapy in this disease state may be related to inhibition of interferon gamma (IFN-γ) and interleukin 15 (IL-15), both of which are activated upstream of TNF.
The annual incidence of EM is estimated at <1%, and it generally impacts people younger than 40, although there’s no obvious association between EM and race, noted Kathryn P. Trayes, MD, of Thomas Jefferson University Hospital in Philadelphia, and co-authors in a 2019 American Family Physician article.
Recurrent EM or persistent EM presents in about 30% of cases and can manifest as isolated oral involvement, or a combination of cutaneous, genital, ocular, and oral disease, any of which may require hospitalization during disease flare-ups. In terms of treatment, continuous antiviral suppression is first-line therapy, but most patients escalate to immunomodulators. “There is no consensus for selection of second-line treatment of chronic EM,” according to Le Cleach’s group.
Also on the low end of incidence is EM tied to vaccination, such as those for hepatitis B or the flu. A 2021 correspondence in the International Journal of Dermatology described a “case of atypical EM occurring shortly after the second dose of BNT162b2 [Comirnaty, Pfizer-BioNTech] with no other evident cause.”
“EM-like reactions have already been linked to Covid-19 infection, both as typical acral lesions in younger individuals and more widespread, atypical lesions in adults,” added Cecilia Buján Bonino MD, of the University Hospital of Santiago de Compostela in Santiago de Compostela, Spain, and co-authors. “Consequently, EM secondary to Covid-19 vaccination would be an expected complication in some cases. It is also notable that this is the first Covid-19 vaccine-related EM having its onset after the second dose.”
Trayes’ group highlighted that while EM “was previously thought…[to be]…on the same pathologic spectrum as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis, it is now accepted that erythema multiforme is a distinct disease.”
Even as a standalone disease, EM is complicated, and “treating CEM can be a challenge… patients with this disease often endure a devastating course without effective treatments after first-line therapy fails,” explained Arturo R. Dominguez, MD, and Samantha N. Lopez, BS, both of the University of Texas Southwestern Medical Center in Dallas, in an accompanying JAMA Dermatology editorial.
They noted that “both of the studies discussed target different immunologic pathways, [but] these pathways are known to be interconnected and are likely linked in the pathogenesis of CEM.”
So does one of the pathways offer a straighter shot to treatment success? Not necessarily, according to Dominguez and Lopez. “It is tempting to speculate that JAK-STAT [signal transducer and activator of transcription] inhibition may be more effective than TNF blockade because of its ability to directly inhibit intralesional pathologic CD8+ and NK cytotoxic T-cells and act through multiple pathways,” they wrote.
Yet the findings from Le Cleach’s group demonstrate that “the pathophysiology of CEM is complex,” Dominguez and Lopez reiterated, and “likely with contributions from numerous pathways involving TNF, IFN-γ, and IL-15 signaling.”
Le Cleach and co-authors conducted a retrospective multicenter cohort study of CEM patients in France who got thalidomide therapy from January 2010 to December 2018. They classified recurrent EM as at least one disease flare annually, while persistent PEM was defined as continuous disease or flares separated by <15 days.
Median patient age was 33 and 57% were female. All participants had experienced failure of at least one previous treatment before starting thalidomide therapy.
The authors reported that at three and 12 months of continuous thalidomide therapy, 83% (95% CI 70% to 95%) and 40% (95% CI 24% to 56%) of patients achieved CR, respectively.
Among patients with persistent EM, 62% achieved CR at 6 months. In the recurrent EM group, some patients did not achieve CR after 12 months of thalidomide, but did see the median number of relapses drop by 67%, from six/year pre-initiation of treatment to two/year on treatment.
For the 23 patients with CR at six months, the median initial dose followed by remission was 50 mg daily, and post-remission maintenance doses were 50 mg daily in eight patients, 50 mg every other day in six patients, and <50 mg every other day in nine patients.
At the time of the study analysis, 29% of the patients were still on treatment for a median 32 months of treatment duration.
The main AEs were asthenia (46%) and neuropathy (40%), although no severe AEs were reported. Notably, the authors found and a thalidomide dose of <50 mg every other day was enough to induce response in 39% of the cohort, but with less neuropathy. However, those patients did have a longer treatment duration.
Study limitations include its retrospective design, single-country setting, and small patient population, although the authors attributed that to the rarity of CEM.
Le Cleach’s group suggested that lenalidomide may be another option as second-line CEM therapy, as was shown in a 2018 JAMA Dermatology observation article.
In the case series from Damsky’s group, all four patients had severe persistent EM with extensive mucocutaneous involvement and had failed numerous other therapies, including antivirals and immunosuppressants.
Mean disease duration in the cases was 21.75 years. Patients received either tofacitinib (5 to 10 mg twice daily) or upadacitinib (15 mg once daily).
In an effort to understand the mechanism of action of the JAK inhibitors in CEM, the authors conducted RNA in situ hybridization analysis of cytokine expression (IFN-γ and IL-15 mRNA levels) in biopsy specimens from 12 EM patients, three of whom were in the study and received tofacitinib.
They reported that in a single patient with a robust improvement following tofacitinib treatment, “RNA sequencing identified IFN-γ and IL-15 as cytokines with activity both highly upregulated at baseline in lesional skin and subsequently suppressed following tofacitinib treatment.”
They also measured IFN-y- and IL-15-positive cells in all the specimens and found significant upregulation of IFN-γ (8.72 cells/mm, 95% CI 2.60 to 14.84) and IL-15 (14.13 cells/mm, 95% CI 0.14 to 28.11) versus normal skin (P=0.008 and P=0.045, respectively).
Again, the small sample size and retrospective design were limitations. But the case study does bolster previous JAK-based research from Damsky and colleague in idiopathic EM and in dermatology in general, and in 2020 an international task force issued a consensus statement in the Annals of Rheumatic Diseases for the use of JAK inhibitors in immune-mediated inflammatory diseases.
-
Second-line thalidomide may be an alternative therapy for chronic erythema multiforme (CEM) based on a 35-person study in which the agent led to complete remission in two-thirds of the patients.
-
A four-patient case series suggested that janus kinase inhibition may be effective in treating persistent EM.
Shalmali Pal, Contributing Writer, BreakingMED™
Le Cleach reported no relationships relevant to the contents of this paper to disclose. A co-author reported relationships with Janssen Cilag, AbbVie, Amgen SAS, Britsol Myers Squibb, Celgene SAS, Galderma, Lilly, and MSD France.
The study by Damsky’s group was supported by the Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
Damsky reported relationships with, and/or support from, a Career Development Award from the Dermatology Foundation, Eli Lilly, Pfizer, Twi Biotech, Pfizer, Advanced Cell Diagnostics/Bio-techne, and EMD/Sigma/Millipore. Co-authors reported support from, and/or relationships with, the Corona Psoriasis Registry, NACE, UCB, LEO Pharma, Novartis, Helsinn, AbbVie, Bristol Meyers Squibb, Arcutis Biotherapeutics, Aclaris Therapeutics, AltruBio, Almirall, Arena Pharmaceuticals, Bioniz Therapeutics, Concert Pharmaceuticals, Dermavant Sciences, Eli Lilly, Incyte, Otsuka/Visterra, Pfizer, Regeneron, Sanofi, Genzyme, and Viela Bio.
Dominguez and Lopez reported no relationships relevant to the contents of this paper to disclose.
Cat ID: 105
Topic ID: 75,105,730,105,192,925