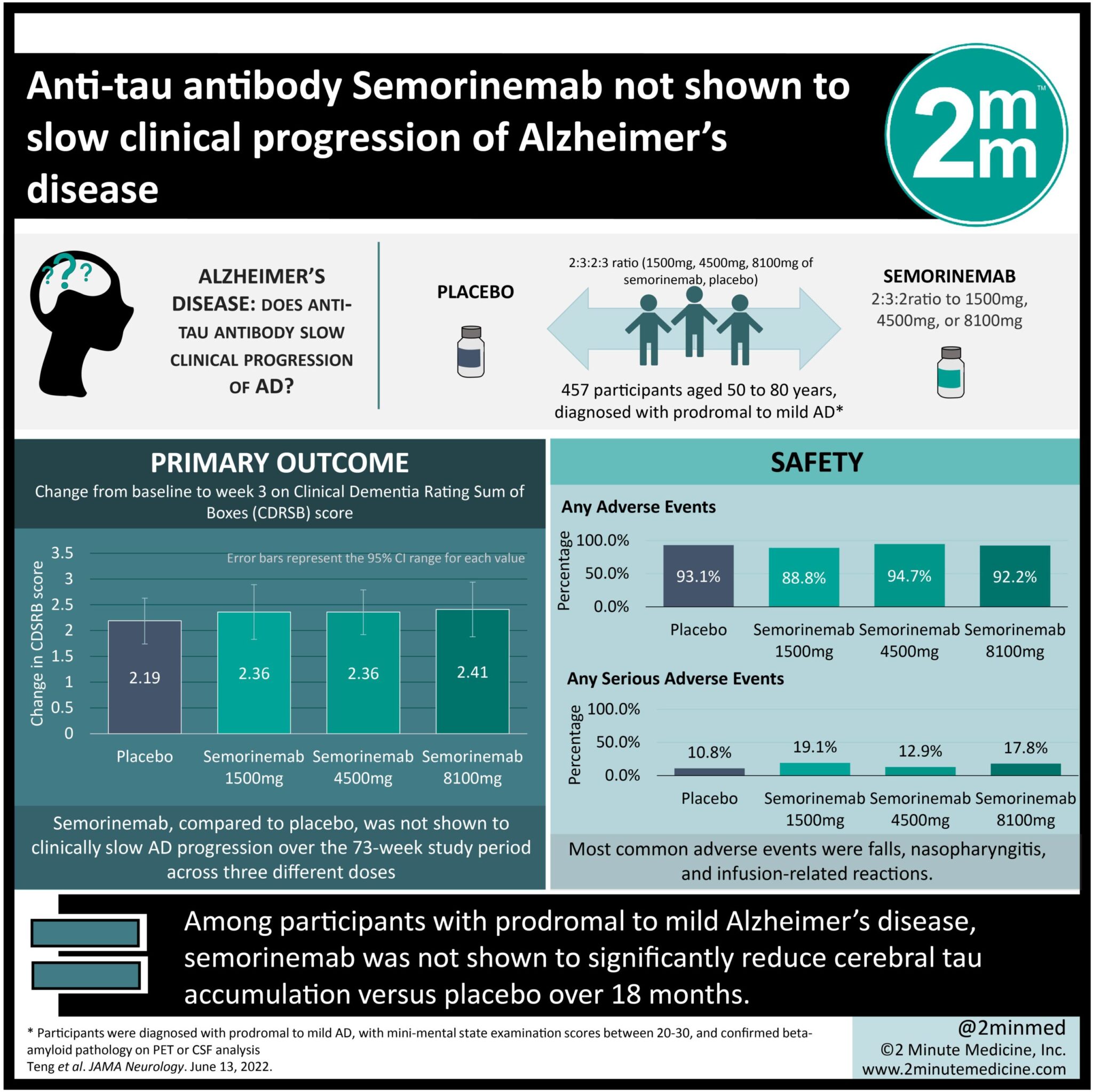
1. In this randomized controlled trial, among 457 participants with prodromal to mild Alzheimer’s disease, semorinemab was not shown to significantly reduce cerebral tau accumulation versus placebo over 18 months.
2. Semorinemab demonstrated a reasonable safety profile comparable to placebo, the most common adverse events being falls, nasopharyngitis and infusion-related reactions.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Alzheimer’s disease (AD) is characterized by neurofibrillary tangles composed of aggregated tau protein. Cerebral tau accumulation has been correlated with disease severity, and is often a target for treatments such as monoclonal antibodies. This phase 2, randomized controlled trial evaluated the safety and efficacy of the monoclonal anti-tau antibody semorinemab in 457 patients with prodromal to mild AD. Over a 73-week blinded study period between 2017 to 2020, participants were randomized to receive intravenous infusions of placebo or semorinemab (1500mg, 4500mg, or 8100mg). Participants were individuals aged between 50 to 80 years, diagnosed with prodromal to mild AD. The main outcomes were change from baseline to week 73 on the Clinical Dementia Rating-Sum of Boxes score (CDRSB), and assessments of the safety and efficacy of semorinemab compared to placebo. In the modified intent to treat cohort, efficacy analyses showed no difference in rates of cerebral tau accumulation or rates of clinical decline of AD between the placebo arm and the semorinemab dose arm at any dosing point. Additionally, similar proportions of participants in the placebo and semorinemab arm experienced adverse events, most of which were mild. These included falls, nasopharyngitis and infusion-related reactions. A notable strength of this study was that it is the first to publish phase 2 clinical data of an anti-tau-monoclonal antibody as a treatment for prodromal to mild AD. Due to the COVID-19 pandemic however, a limitation was that some participants missed doses and/or missed clinical assessments.
Click to read the study in JAMA Neurology
Relevant Reading: Alzheimer’s disease: an update on pathobiology and treatment strategies
In-Depth [randomized controlled trial]: This phase 2, randomized, double-blind, placebo-controlled clinical trial evaluated the safety and efficacy of the monoclonal anti-tau antibody, semorinemab in patients with prodromal to mild AD. The trial was conducted between 2017 and 2020 at 97 sites in North America, Europe and Australia. Individuals aged 50 to 80 years, diagnosed with prodromal to mild AD were included. Participants were randomized in a 2:3:2:3 ratio to either 1500mg, 4500mg, or 8100mg of semorinemab, or placebo. Similar increases in CDRSB were observed in the modified intent-to-treat cohort (n-422; mean [SD] age 69.6 [7.0] years; 235 women [55.7%]) between the placebo group (n=126; = 2.19 [95% CI, 1.74-2.63]) and the semorinemab group (1500mg: n=86, = 2.36 [95% CI, 1.83-2.89]; 4500mg: n=126; = 2.36 [95% CI, 1.92-2.79]; 8100mg: n=84; = 2.41 [95% CI, 1.88-2.94]). A total of 441 participants were placed in the safety-evaluable cohort, and similar proportions experienced adverse events (AEs) in the semorinemab (1500mg: 89 [88.8%]; 4500mg: 132 [94.7%]; 8100mg: 90 [92.2%]) and placebo (130 [93.1%]) group. The most common higher-grade AEs were falls (placebo: 2 [1.5%]; 1500mg: 2 [2.2%]; 4500mg: 2 [1.5%], 8100mg: 1 [1.1%]) and pneumonia (placebo: 1[0.8%]; 1500mg: 1[1.1%]; 4500mg: 1 [1.5%]; 8100mg: 2 [2.2%]). Four deaths were recorded during the blinded portion of the trial (placebo: 2; 4500mg: 1; 8100mg: 1), however investigators did not attribute them to the study drug.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.