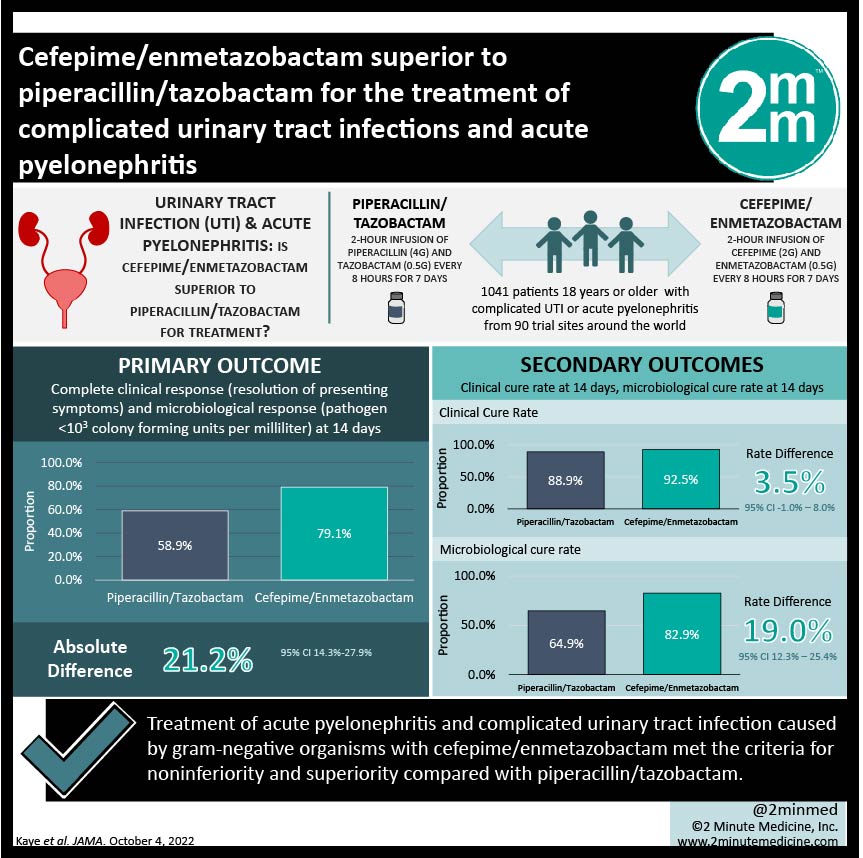
1. In this randomized controlled trial, treatment of acute pyelonephritis and complicated urinary tract infection caused by gram-negative organisms with cefepime/enmetazobactam met the criteria for noninferiority and superiority compared with piperacillin/tazobactam.
2. The rate of treatment-related adverse events in the two groups was comparable, with most adverse events being mild or moderate in severity.
Level of Evidence Rating: 1 (Excellent)
Study Rundown: Piperacillin/tazobactam is a common broad-spectrum antibiotic used to treat serious infections, including complicated urinary tract infections (UTIs) and acute pyelonephritis. However, with frequent use of a single regimen, the risk of antimicrobial resistant bacteria increases and it becomes prudent to switch to alternative agents to avoid this. Cefepime is a fourth-generation cephalosporin which, in combination with the B-lactamase inhibitor enmetazobactam has potent action against B-lactam-producing gram-negative bacteria. This randomized controlled trial compared piperacillin/tazobactam to cefepime/enmetazobactam in a head-to-head comparison in the setting of complicated UTI or pyelonephritis.
In total, 1041 patients were randomized at the start of the trial and 678 were eventually included in the primary analysis. 345 patients were assigned to the cefepime/enmetazobactam group, and 333 were assigned to receive piperacillin/tazobactam. The primary outcome (composite) was achieved by 79.1% of the cefepime/enmetazobactam patients and 58.9% of the piperacillin/tazobactam patients. This indicated that cefepime/enmetazobactam was not only noninferior, but also met the criteria for superiority to piperacillin/tazobactam in this setting. Interestingly, there was no significant difference in the rate of clinical cure between the two study groups, but the cefepime/enmetazobactam group demonstrated a far higher rate of microbiological response than the piperacillin/tazobactam group. The rate of adverse events between the two groups was similar.
This randomized controlled trial by Kaye et al. demonstrated on a global scale that cefepime/enmetazobactam was superior to piperacillin/tazobactam in the treatment of acute pyelonephritis and complicated UTI. These findings are strengthened by several elements of trial design, including randomization of a prospective study and double-blinding. A major limitation of this study is the limited generalizability of findings to clinical practice: patients received intravenous antimicrobials for at least 7 days instead of converting to an oral step down, and the microbiological response was assessed, which is typically not done in clinical practice. Despite this, these findings are important and may influence future decisions regarding antibiotic stewardship in the setting of complicated UTIs and acute pyelonephritis.
Click here to read this study in JAMA
Click to read an accompanying editorial in JAMA
Relevant reading: An update on the management of urinary tract infections in the era of antimicrobial resistance
In-Depth [randomized controlled trial]: A double-blinded, multicenter, randomized controlled trial was conducted. Participants from 90 trial sites around the world were included. Eligible individuals were adults with either complicated UTI or acute pyelonephritis as defined by prespecified criteria. Patients were randomized to receive either cefepime/enmetazobactam or piperacillin/tazobactam using a computer-generated randomizer in a 1:1 ratio. Patients received a 2-hour infusion of either cefepime (2g) and enmetazobactam (0.5 g) or piperacillin (4 g) and tazobactam (0.5 g) every 8 hours for 7 days. Patients with positive blood cultures at baseline were treated for 14 days total. The primary outcome was complete clinical response (resolution of presenting symptoms) and microbiological response (pathogen <103 colony forming units per milliliter) at 14 days.
Patients were included between September 2018 and November 2019. In total, 1041 patients were randomized in this study and 1034 received at least one dose of the study drugs. 678 individuals were included in the primary analysis, including 349 who had acute pyelonephritis, 148 who had a complicated UTI with a removable source of infection, and 181 who had a UTI without a removable source of infection. The most common pathogen was Erischeria coli (76% of cases).
The primary composite outcome of complete clinical and microbiological response was achieved at day 14 by 79.1% of the patients in the cefepime/enmetazobactam group and 58.9% of patients in the piperacillin/tazobactam group. The absolute difference was 21.2% (95% confidence interval 14.3-27.9%). The clinical cure rate was not different between the two groups: 92.5% in the cefepime/enmetazobactam group and 88.9% in the piperacillin/tazobactam group, rate difference was 3.5% (95% confidence interval -1.0-8.0%). However, the rate of microbiological cure was significantly higher in the cefepime/enmetazobactam group (82.9%) compared to the piperacillin/tazobactam group (64.9%). The cure rate difference was 19.0% (12.3%-25.4%) at day 14. The rate of adverse events in the cefepime/enmetazobactam group was 50.0% and 44.0% in the piperacillin/tazobactam group, which was not significantly different.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.