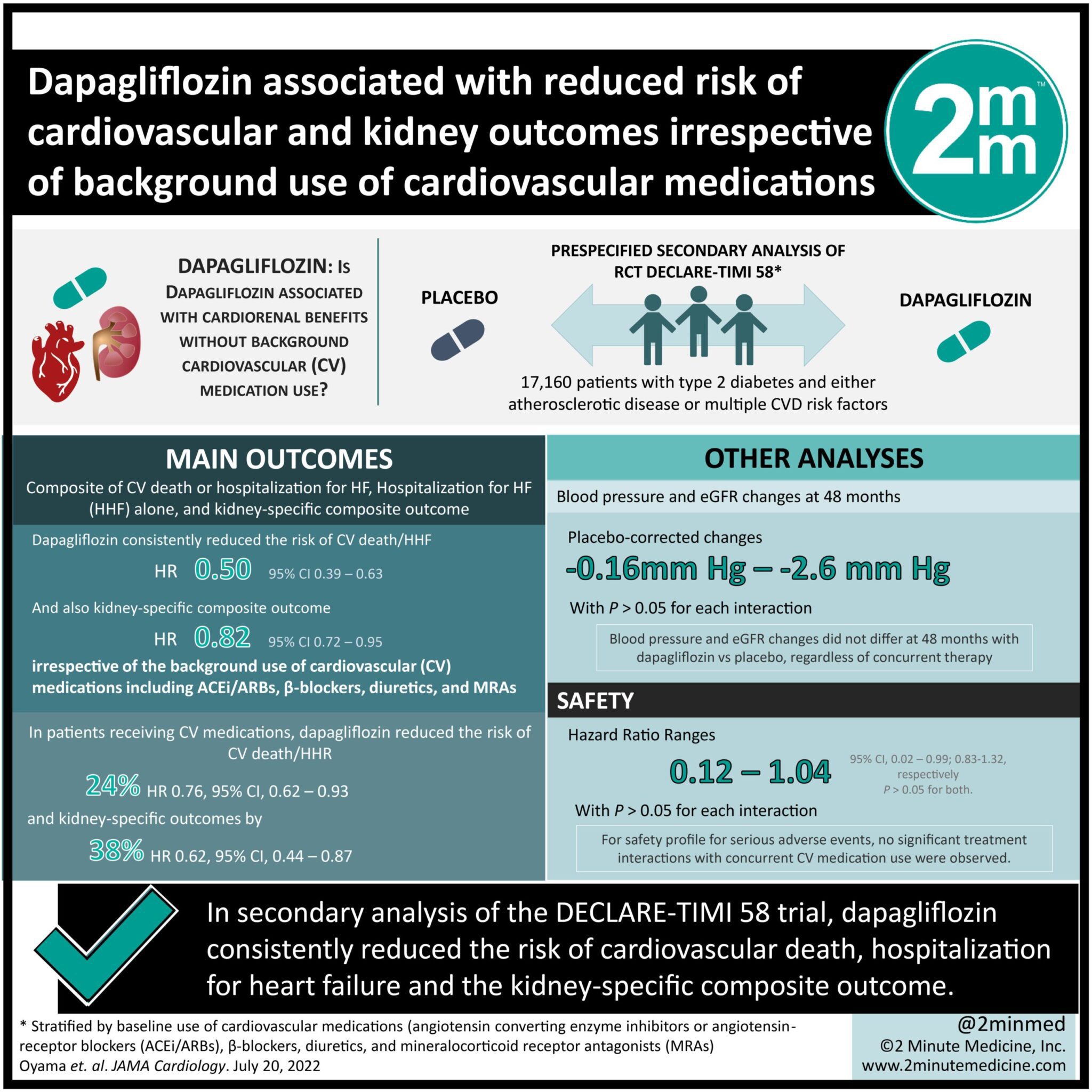
1. In this secondary analysis of the DECLARE-TIMI 58 trial, among 17,160 patients, dapagliflozin consistently reduced the risk of cardiovascular death, hospitalization for heart failure and the kidney-specific composite outcome.
2. In patients receiving angiotensin converting enzyme inhibitors or angiotensin-receptor blockers with both β-blockers and diuretics, dapagliflozin reduced the risk of cardiovascular death and hospitalization for heart failure by 24% and kidney-specific composite outcome by 38%.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that blocks glucose reabsorption in the proximal tubule of the kidneys and promotes glucosuria. This class of medication has been proven to reduce cardiovascular (CV) events and kidney disease progression, specifically in patients with type 2 diabetes. In this prespecified secondary analysis of the DECLARE-TIMI 58 trial, the objective was to assess whether cardiorenal efficacy and safety of dapagliflozin were consistent with, and without CV medication use in the background. The DECLARE-TIMI 58 trial was a randomized trial of dapagliflozin vs placebo among 17,160 patients with type 2 diabetes, and either atherosclerotic disease or multiple CV disease risk factors. Patients in this study were stratified by their baseline use of: angiotensin converting enzyme inhibitors or angiotensin-receptor blockers (ACEi/ARBs), β-blockers, diuretics, and mineralocorticoid receptor antagonists (MRAs), all medications commonly used for heart failure (HF) and kidney disease in patients with type 2 diabetes. The main outcomes were composite of CV death or hospitalization for HF, hospitalization for heart failure (HHF) alone, and a kidney specific composite outcome. Among 17,160 patients, dapagliflozin consistently reduced the risk of CV death/HHF, HHF alone and the kidney-specific composite outcome, regardless of use of background medications. In patients receiving ACEi/ARBs with both β-blockers, and diuretics, dapagliflozin reduced the risk of CV death/HHF and kidney-specific composite outcome by 24%, and 38%, respectively. This timely analysis demonstrated that the safety profile of dapagliflozin was consistent in patients regardless of their CV medication use. A limitation of the study was that since the dose of each medication was not recorded, treatment interactions across a range of doses could not be examined.
Click to read the study in JAMA Cardiology
Relevant Reading: Dapagliflozin and cardiovascular outcomes in type 2 diabetes
In-Depth [randomized clinical trial]: This study is a prespecified secondary analysis of the randomized clinical trial DECLARE-TIMI 58 conducted between 2013 to 2018, which compared dapagliflozin vs placebo among 17,160 patients with type 2 diabetes and either atherosclerotic disease or multiple CVD risk factors. Among the 17,160 patients, 13,950 (81%) used ACEi/ARBs, 9030 (53%) used β-blockers, 6205 (36%) used diuretics, and 762 (4%) used MRAs at baseline. Blood pressure and eGFR changes did not differ at 48 months with dapagliflozin vs placebo, regardless of concurrent therapy (placebo-corrected change, -1.6mmHg [95% CI, -4.2 to 1.0] to -2.6mmHg [95% CI, -3.3 to -2.9]; P > .05 for each interaction). Dapagliflozin consistently reduced the risk of CV death/HHF, and kidney-specific composite outcome irrespective of the background use of CV medications including ACEi/ARBs, β-blockers, diuretics, and MRAs (hazard ratio [HR] range: HR, 0.50; 95% CI, 0.39-0.63; to HR, 0.82; 95% CI, 0.72-0.95; P > .05 for each interaction). In patients who were concurrently on all the following medications: ACEi/ARBs, β-blockers and diuretics (n= 4243), dapagliflozin reduced the risk of CV death/HHR and kidney specific outcomes by 24% (HR, 0.76; 95% CI, 0.62-0.93) and 38% (HR, 0.62; 95% CI, 0.44-0.87), respectively. Dapagliflozin consistently reduced the risk of kidney specific composite outcome regardless of the concurrent use of CV medications except for diuretics (P > .05 for each interaction). In terms of the safety profile for serious adverse events, no significant treatment interactions were found for concurrent CV medication use for adverse events of volume depletion, acute kidney injury, or hyperkalemia (range: HR, 0.12; 95% CI, 0.02-0.99; to HR, 1.04; 95% CI, 0.83-1.32; P > .05 for each interaction).
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.