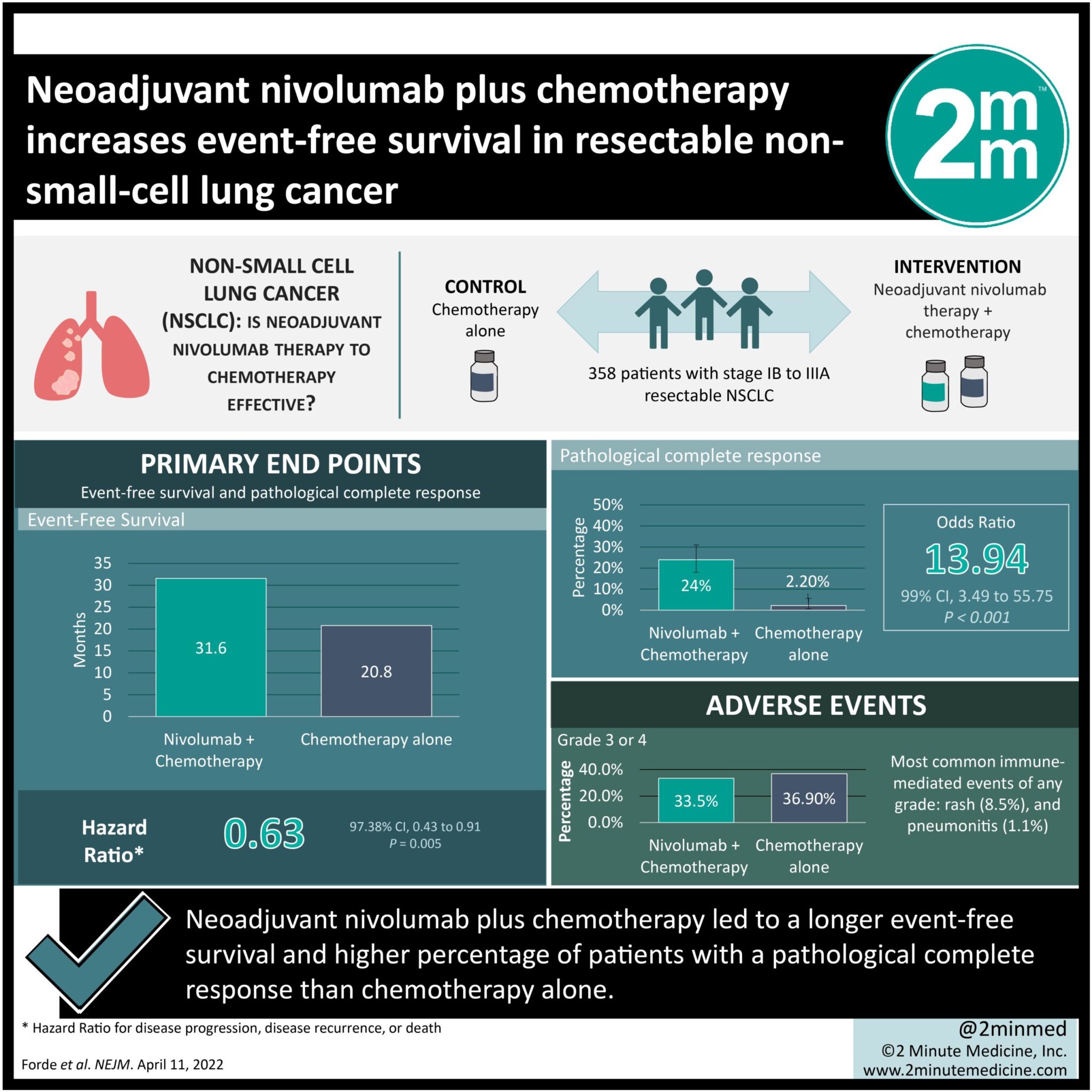
1. Neoadjuvant nivolumab plus chemotherapy led to a longer event-free survival and higher percentage of patients with a pathological complete response than chemotherapy alone.
2. Addition of nivolumab as a neoadjuvant did not increase the incidence or severity of adverse events, nor did it impact the feasibility of surgery.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The addition of neoadjuvant immunotherapy to a chemotherapy or surgery regimen has proven to be effective in some cancers. This study explored the efficacy and safety of nivolumab (an anti-programmed death 1 antibody) as a neoadjuvant therapy for patients with resectable non-small-cell lung cancer (NSCLC). These patients were randomly assigned to receive neoadjuvant nivolumab and chemotherapy, or chemotherapy alone, both followed by surgery. Median event-free survival (EFS) in the nivolumab group was longer and the percentage of patients with a pathological complete response was higher. These results were consistent across most subgroups, with the highest magnitude of benefit in EFS being in stage IIIA disease patients. More patients in the nivolumab group underwent definitive surgery than the chemotherapy group. In the nivolumab group, median duration of surgery was shorter, minimally invasive procedures were more common, and pneumonectomies were less common. The incidence of adverse events was not increased with the addition of neoadjuvant nivolumab, and occurrence of grade 3 or 4 treatment-related adverse events were not significantly different between both groups. Most common grade 3 or 4 adverse events across both groups include neutropenia and decreased neutrophil count. Limitations to this study are its predominantly male patients and that it does not discuss the necessity of adjuvant therapy in this treatment regimen. The strengths of this study are that it has limited bias given its design and that it was able to highlight the clinical benefit of neoadjuvant nivolumab in lung cancer. Overall, addition of neoadjuvant nivolumab is a viable treatment option in resectable NSCLC.
Click to read the study in NEJM
Click to read an accompanying editorial in: NEJM
Relevant Reading: Early stage resectable non-small cell lung cancer: is neoadjuvant immunotherapy the right way forward?
In-Depth [randomized control trial]: This international, phase III trial randomly assigned 358 patients with stage IB to IIIA resectable NSCLC into two groups before having them undergo resection: 179 in the neoadjuvant nivolumab plus chemotherapy group and 179 in the chemotherapy alone group. Median EFS was 31.6 months in the nivolumab group and 20.8 months in the chemotherapy group (hazard ratio [HR] for disease progression, disease recurrence, or death, 0.63; 97.38% confidence interval [CI], 0.43 to 0.91; P=0.005). Percentage of patients with pathological complete response was 24.0% in the nivolumab group and 2.2% in the chemotherapy group (odds ratio, 13.94; 99% CI, 3.49 to 55.75; P<0.001). In the nivolumab and chemotherapy groups, 83.2% and 75.4% of patients underwent surgery, respectively. Incidence of adverse events of any cause in the nivolumab and chemotherapy groups were 92.6% and 97.2%, respectively. Incidence of grade 3 or 4 treatment-related adverse events were 33.5% and 36.9%, respectively, with neutropenia (8.5% vs. 11.9%, respectively) and decreased neutrophil count (7.4% and 10.8%, respectively) being the most common. Incidence of immune-mediated events in the nivolumab group were low and mainly consisted of grades 1 and 2. Most common immune-mediated events of any grade were rash (8.5%) and pneumonitis (1.1%). Overall, this study showed that nivolumab as a neoadjuvant therapy significantly increased EFS and yielded a higher percentage of pathological complete response than chemotherapy alone.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.