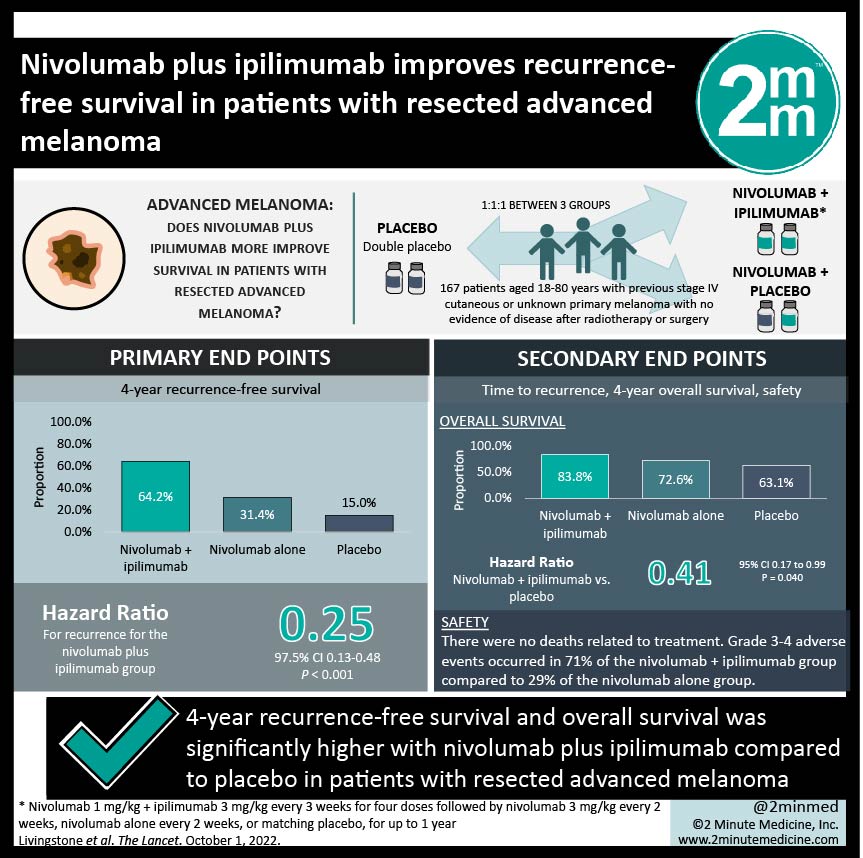
1. 4-year recurrence-free survival was significantly higher with nivolumab plus ipilimumab compared to placebo.
2. Overall survival was significantly higher in nivolumab plus ipilimumab compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Melanoma is a form of skin cancer with the aggressive potential to metastasize to other organs of the body. This study assessed the use of immune checkpoint inhibitors in patients with previously resected stage IV melanoma and no current evidence of disease. Patients were randomly assigned to either a regimen of nivolumab plus ipilimumab, nivolumab alone, or placebo, for up to 1 year. Both the nivolumab-ipilimumab and nivolumab monotherapy groups showed significantly improved 4-year recurrence-free survival compared to placebo. Overall survival was additionally improved in the nivolumab plus ipilimumab group compared to placebo. Adverse events were consistent with previously described for the two drugs. Limitations of the IMMUNED trial include the study size. Nonetheless, the IMMUNED trial demonstrates the clinical benefit of nivolumab and ipilimumab in patients with stage IV melanoma with no evidence of disease.
Click to read the study in The Lancet
Relevant Reading: Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial
In-Depth [randomized controlled trial]: IMMUNED was a phase 2 drug trial that took place across 20 academic medical centres in Germany. Patients were eligible if they were aged 18-80 years and had previous stage IV cutaneous or unknown primary melanoma with no evidence of disease after radiotherapy or surgery. A total of 167 patients were randomized 1:1:1 to either nivolumab plus ipilimumab (n=56), nivolumab plus ipilimumab-matching placebo (n=59), or double placebo control (n=52). Treatment regimen was nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab 3 mg/kg every 2 weeks, nivolumab alone every 2 weeks, or matching placebo, for up to 1 year. The primary endpoint was recurrence-free survival. The median follow-up time was 49.2 months. The 4-year recurrence-free survival was highest in the nivolumab plus ipilimumab group (64.2%), followed by nivolumab alone (31.4%), and then placebo (15.0%). Compared to placebo, the hazard ratio for recurrence for the nivolumab plus ipilimumab group was 0.25 (97.5% CI 0.13-0.48, p<0.0001). For overall survival between nivolumab plus ipilimumab group versus placebo, the hazard ratio was 0.41 (p=0.04). There were no deaths related to the treatment. Grade 3-4 adverse events occurred in 71% of the nivolumab plus ipilimumab group compared to 29% of the nivolumab alone group.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.