1. In this randomized controlled trial, oral antimicrobial prophylaxis was reported to reduce the incidence of surgical site infections (SSI) when compared to a placebo in patients undergoing elective colorectal surgery.
Evidence Rating Level: 1 (Excellent)
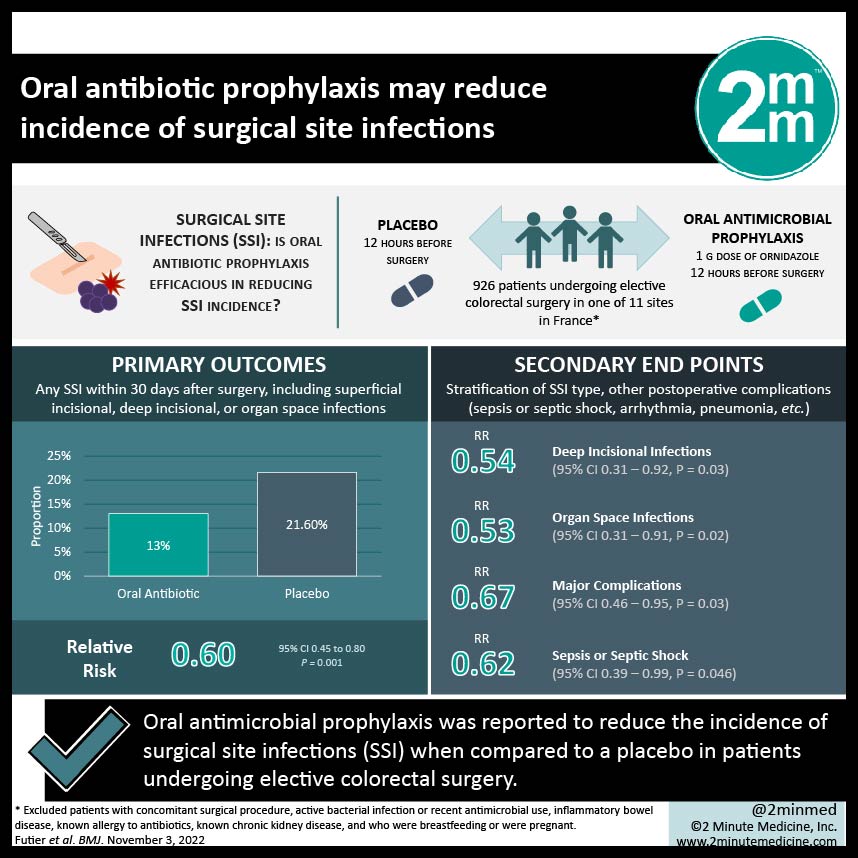
Study Rundown: Surgical site infection (SSI) is one of the most common healthcare-related infections, especially in patients undergoing colorectal surgery with reported incidence rates of SSI up to 26%. Current guidelines recommend intravenous antimicrobial therapy against both aerobic and anaerobic bacteria to prevent SSI following colorectal surgery. The efficacy of oral antimicrobial therapy has been studied, and a recent meta-analysis reported a reduction of SSI by more than 50%, but current existing evidence remains quite limited. This study is a multicenter, randomized, double-blind, placebo controlled trial that was conducted in 11 sites in France. Patients were randomized in a 1:1 ratio to receive either a 1g dose of ornidazole or a placebo prior to surgery. Otherwise, all patients received a 2nd generation cephalosporin with anaerobic activity (cefoxitin 2g) at least 30 minutes before skin incision as well, and intraoperatively if the procedure lasted 2 hours or longer following current guidelines. Included were patients scheduled for elective laparoscopic or open colorectal surgery. The primary outcome analyzed in this study was any SSI within 30 days after surgery, including superficial incisional, deep incisional, or organ space infections. Secondary outcomes included stratification of SSI type, as well as other postoperative complications. Between May 2016 and August 2019, 926 patients were randomized, with 463 patients receiving ornidazole and 463 patients receiving a placebo. With respect to the primary outcome, the rate of SSI within 30 days after surgery was significantly lower in the oral prophylaxis group compared to the placebo group; the incidence of SSI was 13% (60/463) in the oral prophylaxis group, and 21.6% (100/463) in the placebo group (ARR 0.62, 95% CI 0.44-0.46, P=.001). Concerning secondary outcomes, significant differences were reported in deep and organ space infections, major complications within 30 days after surgery, and sepsis or septic shock. Of note, there was no significant difference between the two groups in death at 30 days. The findings from this study suggest that adding a single dose of 1g of ornidazole 12 hours before surgery as an adjunct to intravenous antibiotics resulted in a significantly lower rate of SSI compared to a placebo. A major strength of this study is the large sample size; however, limitations include the use of cefoxitin limiting external validity given increasing anaerobic resistance and decreased generalization of results to excluded patient populations who often require colorectal surgery including patients with inflammatory bowel disease. This study is an important step towards reducing the extremely high incidence rates of SSI, especially in elective colorectal surgery, and developments in antimicrobial prophylaxis will lead to decreases in patient morbidity and associated healthcare costs.
Click to read the study in BMJ
Relevant Reading: Strategies for Antibiotic Administration for Bowel Preparation Among Patients Undergoing Elective Colorectal Surgery
In-Depth [randomized controlled trial]: This study analyzed the relationship of oral antimicrobial prophylaxis versus placebo in preventing SSIs in patients undergoing elective colorectal surgery. Included in the analysis were patients undergoing elective colorectal surgery in one of 11 sites in France, and excluded were patients with a concomitant surgical procedure, an active bacterial infection or recent antimicrobial use, inflammatory bowel disease, a known allergy to antibiotics, known chronic kidney disease, and who were breastfeeding or were pregnant. 926 patients were randomized in a 1:1 ratio to receive a placebo or oral antimicrobial prophylaxis, with 463 (50%) patients in each group. Baseline characteristics were similar between the two groups, except for the placebo group having more men and patients with coronary artery disease. 593 patients (64%) underwent colon resection, and 333 patients (36%) underwent rectal resection. 685 (74%) procedures were performed laparoscopically. Concerning the primary outcome studied, patients who received oral antimicrobial prophylaxis had a significantly lower rate of SSI compared to the placebo group (P=.001). In terms of secondary outcomes, significant differences existed for deep incisional infection (4.8% vs 8.0%; RR 0.54, 95% CI 0.31-0.92; P=.03), organ space infections (5.0% v 8.4%; RR 0.53, 95% CI 0.31-0.91; P=.02), major complications (RR 0.67, 95% CI 0.46-0.95; P=.03), and sepsis or septic shock (P=.046). Of note, there was no significant difference between the two groups for death within 30 days (P=.27). Overall, this randomized controlled trial suggests that oral antimicrobial prophylaxis resulted in reduced rates of SSI in patients undergoing elective colorectal surgery.