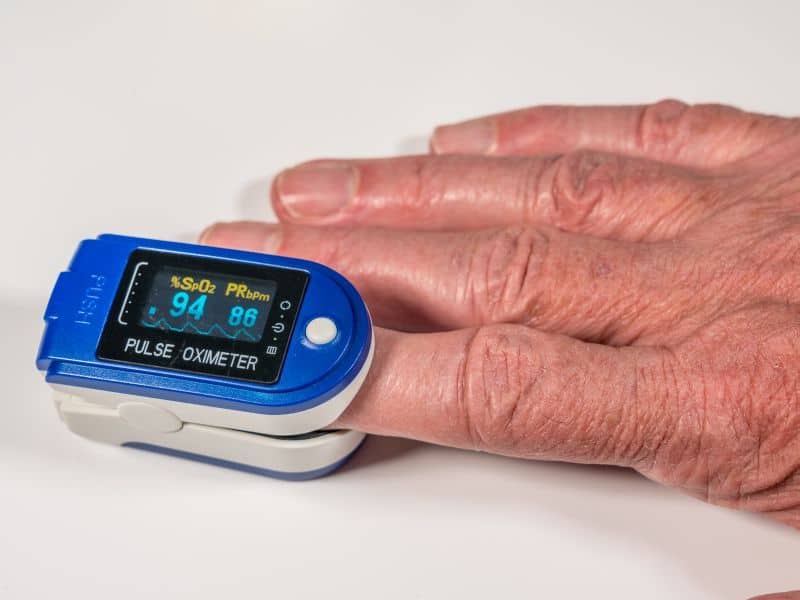
TUESDAY, Feb. 23, 2021 (HealthDay News) — The U.S. Food and Drug Administration is warning against reliance on pulse oximeters for diagnosis and treatment decisions for COVID-19 and other conditions.
In a safety communication released Friday, the agency said that “pulse oximetry is useful for estimating blood oxygen levels,” but there is evidence of limitations and a risk for inaccuracy with the use of pulse oximeters. The FDA suggests instead that patients “pay attention to all signs and symptoms of their condition and communicate any concerns to their health care provider.”
The safety communication follows a study published in the New England Journal of Medicine in December suggesting that pulse oximeters may be less accurate when used in people with dark skin pigmentation. In that study, researchers found that compared with White patients, Black patients had nearly three times greater frequency of occult hypoxemia that was not detected by pulse oximetry.
The FDA recommends that health care providers consider factors that can affect the accuracy of pulse oximeter reading such as poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, and fingernail polish. The agency also recommends referring to the device labeling or manufacturer website to learn more about the accuracy of a particular pulse oximeter and sensor as different brands have different accuracy levels. Pulse oximeter readings should be used as an estimate of blood oxygen saturation, and when possible, diagnosis and treatment decisions should be based on trends over time rather than absolute thresholds of pulse oximeter readings.
The FDA is evaluating published data and premarket data to determine how factors such as skin pigmentation may affect the accuracy of pulse oximetry. The agency asks that any problems with pulse oximeters are reported through the MedWatch program.
Copyright © 2020 HealthDay. All rights reserved.