When the American Association for the Study of Liver Diseases (AASLD) kicks off its 2021 meeting on Nov. 12, it will be streaming to registrants worldwide, but it will not be presenting the latest findings in hepatology to a live audience in Anaheim as was originally planned.
Blame it on Covid-19 and the Delta variant. Although AASLD had originally planned a hybrid meeting offering both live and virtual presentations, a change in plans was announced in this message:
“With increasing uncertainties around variants of the Covid-19 virus, the AASLD Governing Board has made the decision to cancel plans for the in-person component of The Liver Meeting in Anaheim. As medical professionals, it is imperative that we put the health and safety of our participants first, and developments surrounding the virus and its variants have raised significant concerns about safely bringing a large group of people together.” Those who registered to attend the in-person meeting are being refunded the in-person registration costs and will be able to remain registered for the Liver Meeting Digital Experience.
“We are sharpening our focus on the new and innovative ways we can present the content our participants desire most—finding creative solutions to enhance digital networking sessions; exploring opportunities to enrich poster presentations; and of course—delivering the trademark educational programming that is the hallmark of The Liver Meeting. As we learned from The Liver Meeting Digital Experience 2020, our community is deeply invested in the education provided by our annual event, and we are certain this year’s meeting will be no exception,” the AASLD meeting planners said in a message to members.
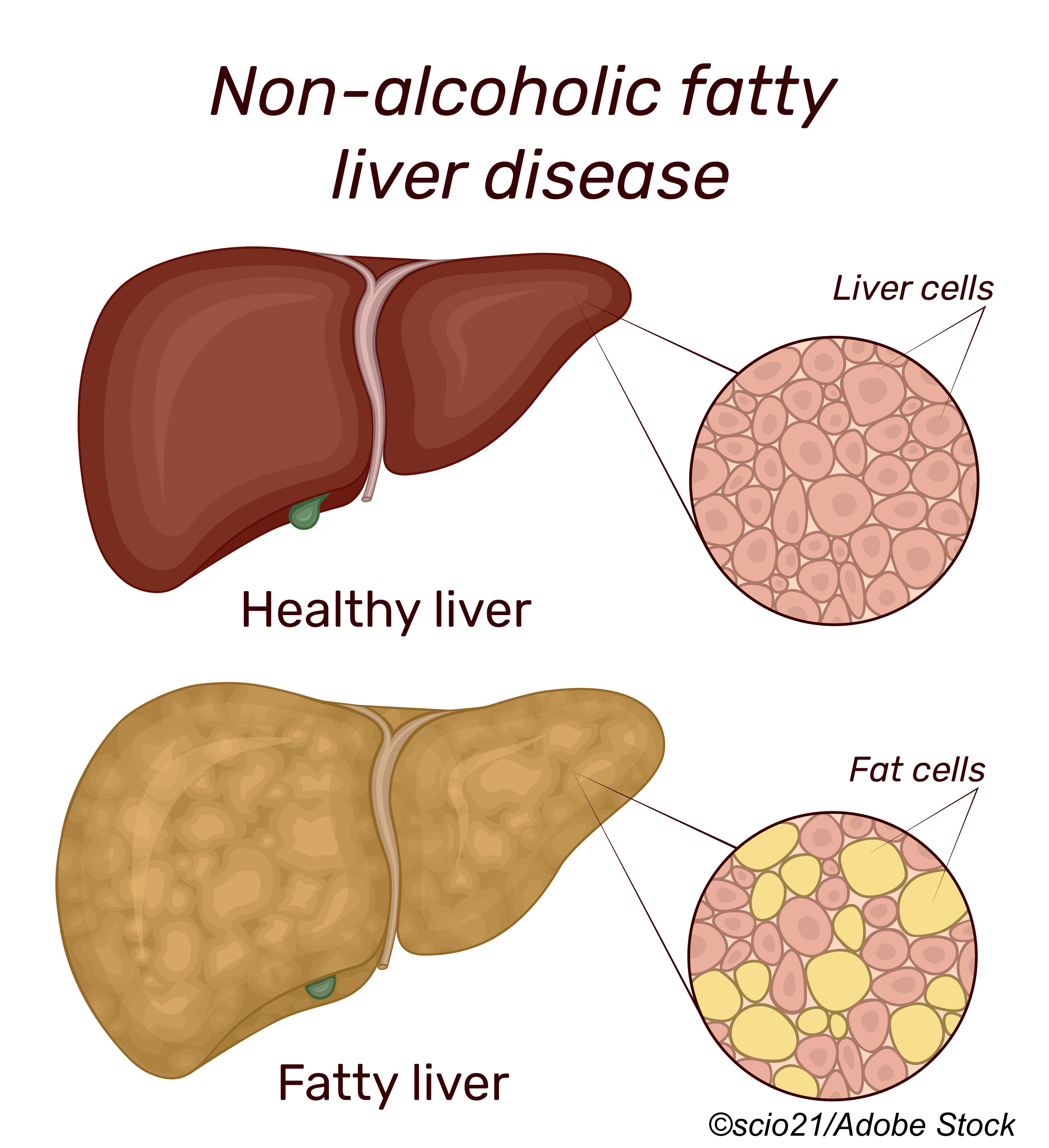
But live or not, The Liver Meeting will have a packed agenda, including several presentations on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) that will focus on current clinical strategies, as well as investigational therapies.
A three-part, full-day, postgraduate course slated for Saturday, Nov. 13, will cover managing the epidemic of fatty liver from obesity and alcohol with presentations from 25 faculty members.
And late-breaking clinical trials scheduled to be presented will include first-in-human data from a trial titled, “CB4211, a novel analog of MOTS-c, improves markers of liver injury and metabolism in obese subjects with nonalcoholic fatty liver disease: A multicenter, double-blind, randomized, placebo-controlled study.” The investigational agent CB4211 is a first-in-class mitochondria-based therapeutic (MBT) under development for the treatment of NASH and obesity.
Other research expected to generate buzz in the virtual hallway include:
- Phase Ib data on EDP-514, a core inhibitor, for hepatitis B.
- Phase Ib/IIa data on VTP-300, a therapeutics viral vaccine, for hepatitis B.
- Phase II data on rusfertide (PTG-300) in hereditary hemochromatosis.
Peggy Peck, Editor-in-Chief, BreakingMED™
Cat ID: 111
Topic ID: 77,111,730,111,188,230,192,925,229