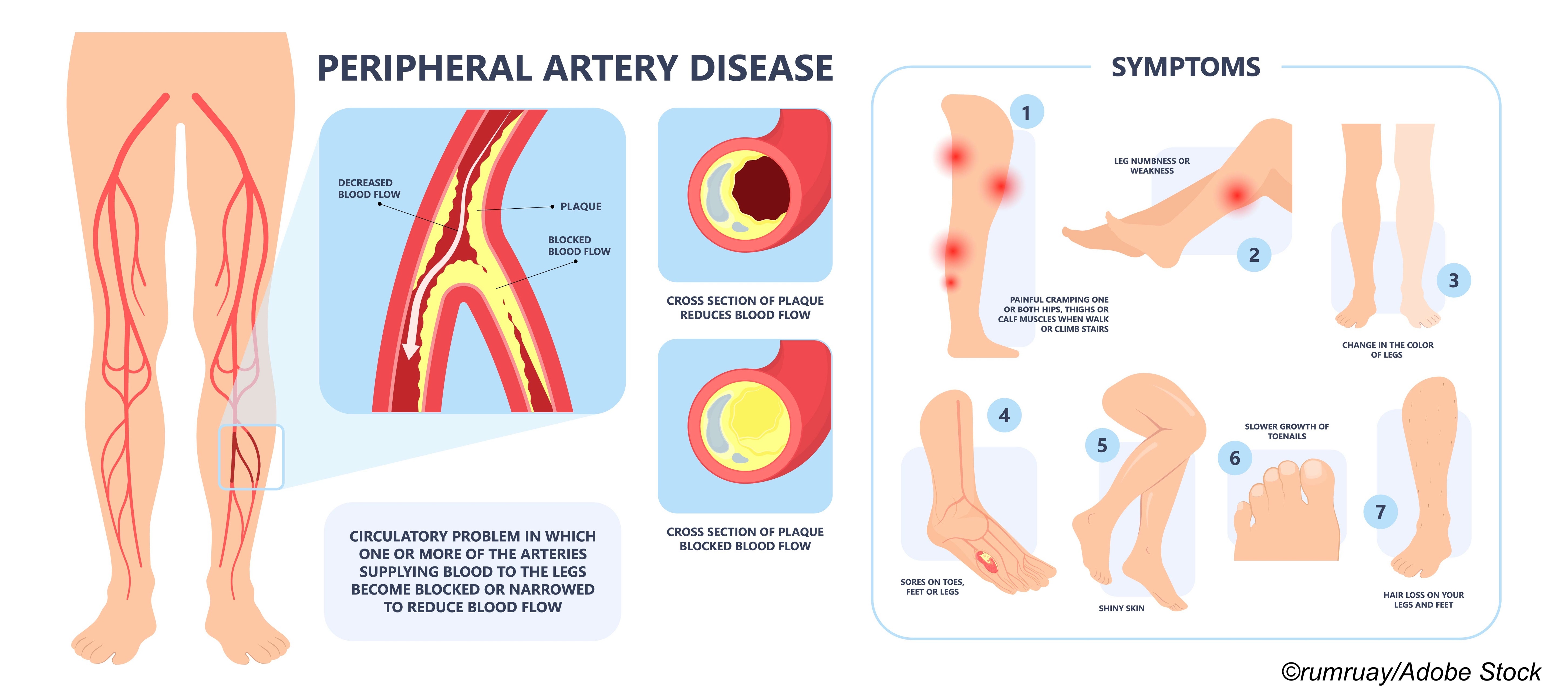
When a 2018 meta-analysis of 28 trials reported “a significant relationship” between exposure to paclitaxel via a drug-eluting stent used to treat peripheral artery disease (PAD) and excess mortality, the FDA responded by first issuing a warning letter, followed by a second letter urging doctors to avoid paclitaxel drug-coated devices (DCDs). The net result was a significant reduction in DCD use, despite the fact that the excess mortality didn’t surface until 2 years after the DCDs were placed in index limbs.
The likelihood of paclitaxel either directly improving or worsening outcome so long after a single index exposure defies common sense, Valentin Fuster, MD, MPH, observed in a podcast that accompanied publication of a new analysis of data from the VOYAGER PAD trial published in the Journal of the American College of Cardiology.
Fuster, who is the editor of JACC, director of Mount Sinai Heart, and Physician-in-Chief Mount Sinai Hospital, said he hopes the findings from this latest analysis by Connie N. Hess, MD, MHS, of the Department of Medicine, Division of Cardiology, University of Colorado School of Medicine, Aurora, Colorado, and colleagues will be convincing enough to put the bad DCD news genie back in the bottle.
VOYAGER-PAD enrolled 4,316 patients and treated 1,342 (31.2%) with DCDs. About 28% of patients were women and the average age of patients was late 60s.
After 31 months follow-up, the DCDs were associated with a slight reduction in mortality risk (HR: 0.95; 95% CI 0.83-1.09 P =0.49). Moreover, in a weighted comparison to non-drug coated devices, DCD significantly reduced the need for repeat revascularization procedures: 21.5% versus 24.6% (HR: 0.84, 95% CI 0.76-0.92, P=0.0003). At 3 years, the number needed to treat to avoid one unplanned index limb revascularization was 33.
Looking at the secondary outcome of major adverse limb events (MALE), again there was no measurable difference: 6.5% MALE with DCD versus 6.3% with non-DCD (HR, 1.09; 95% CI 0.82-1.41) P=0.60.
In an editorial, Thomas Zeller, MD, and Tanja Böhme, MD, of the University Heart Center Freiburg–Bad Krozingen, Bad Krozingen, Germany, pointed out that the findings reported by Hess et al mirror those of “a recent interim analysis of the SWEDPAD (Swedish Drug-Elution Trial in Peripheral Arterial Disease) study.”
SWEDPAD investigators reported that at one year, “all-cause mortality was 10.2% (117 patients) in the drug-coated–device group and 9.9% (113 patients) in the uncoated-device group. During the entire follow-up period, there was no significant difference in the incidence of death between the treatment groups among patients with chronic limb-threatening ischemia (33.4% [249 patients] in the drug-coated–device group and 33.1% [243 patients] in the uncoated-device group) or among those with intermittent claudication (10.9% [44 patients] and 9.4% [38 patients], respectively).”
Of note, the majority of patients in SWEDPAD had chronic limb-threatening ischemia (n=1,480) versus intermittent claudication (n=809), but in VOYAGER PAD, revascularization was most often performed for claudication: 85.2% of DCD procedures and 78.5% of non-DCD procedures.
Zeller and Böhme noted that there are many factors that may contribute to mortality: ” …mainly including prescribed medication and in particular the patient’s drug adherence to secondary preventive medication, their sex, and the patient’s adherence to exercise programs. No RCT or real-world study considered interaction of these variables with the outcome after paclitaxel exposure. An analysis of unselected all-comer administrative data from Germany identified female sex and lesion level above the knee as predictors for reduced mortality following DCD treatment. Interestingly, females with no prior revascularization history were especially positively affected by paclitaxel exposure. The investigators concluded that this could ’be a sign of improved management in a subgroup of patients who exhibited insufficient prior care.’”
Hess and colleagues wrote that in VOYAGER PAD, there was close to 99.6% ascertainment of mortality and follow-up beyond the 2-year time point at which mortality became apparent in the 2018 meta-analysis.
“Our data demonstrated no mortality or MALE safety signal associated with DCDs. In the unadjusted analysis, lower, not higher, mortality with DCD use was observed; this was likely due to differences in the groups (e.g., less treatment of critical limb ischemia and greater use of medical therapies, such as statins, in patients treated with DCDs). After weighting, the adjusted HR was 0.95 with a narrow CI, the upper limit of which excluded even a 10% excess mortality risk associated with DCDs… In VOYAGER PAD, only 6 patients were lost to follow-up, and our results would not change, even assuming DCD treatment and deaths for these lost patients. Although increased risk of mortality was observed among the DES versus BMS subgroups (17.5% versus 13.4%), the numbers were small and not significantly different, as evidenced by the neutral HR and wide CIs. Furthermore, although there was a safety concern that infrapopliteal paclitaxel use could contribute to subsequent amputation (26), we found no association between DCDs and increased risk of MALE,” Hess et al wrote.
In their editorial, Zeller and Böhme pointed out that the excess mortality reported in the 2018 meta-analysis has not been confirmed and is unlikely to be, as “[d]ue to the low event rate and multiple confounders, it will be hard to confirm or finally exclude this mortality risk even in a dedicated RCT.”
Which brings us back to Fuster’s closing words in his podcast: “Frankly, the take home message from the combination of the study today and common sense tells me to be comfortable with paclitaxel-coated devices for lower extremity revascularization. And I hope you feel the same.”
-
In patients with PAD who underwent lower extremity revascularization using paclitaxel drug coated devices (DCDs), there was no excess mortality and less restenosis requiring repeat revascularization procedures.
-
Randomized trials are needed to more completely evaluate the long-term safety and efficacy of DCD-based lower extremity revascularization in patients with intermittent claudication or chronic limb ischemia.
Peggy Peck, Editor-in-Chief, BreakingMED™
VOYAGER PAD was funded by Bayer and Janssen Pharmaceuticals. A Pan-Industry Consortium (Medtronic, Boston Scientific, Cook, Philips, Bard, Surmodics, TriReme) provided funding through a grant to CPC Clinical Research to support statistical analyses performed at CPC Clinical Research.
Hess received grants to CPC Clinical Research from Bayer, Janssen, and Amgen during the conduct of the study; and has received grants from Merck and Amgen outside the submitted work.
Zeller has received honoraria from Abbott Vascular, Bayer, Biotronik, Boston Scientific Corp., Cook Medical, CSI, Efemoral, Gore &Associates, Medtronic, Philips, Shockwave, Veryan, Vesper Medical, and VentureMed; has received institutional research or clinical trial funding from Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Medtronic, Philips, Terumo, TriReme, Shockwave, Med Alliance, Intact Vascular, B. Braun, CSI, Boston Scientific, University of Jena, Pluristem, Philips, and PQ Bypass; and holds common stock in QT Medical.
Böhme has reported that she has no relationships relevant to the contents of this paper to disclose.
Cat ID: 206
Topic ID: 74,206,730,206,192,925