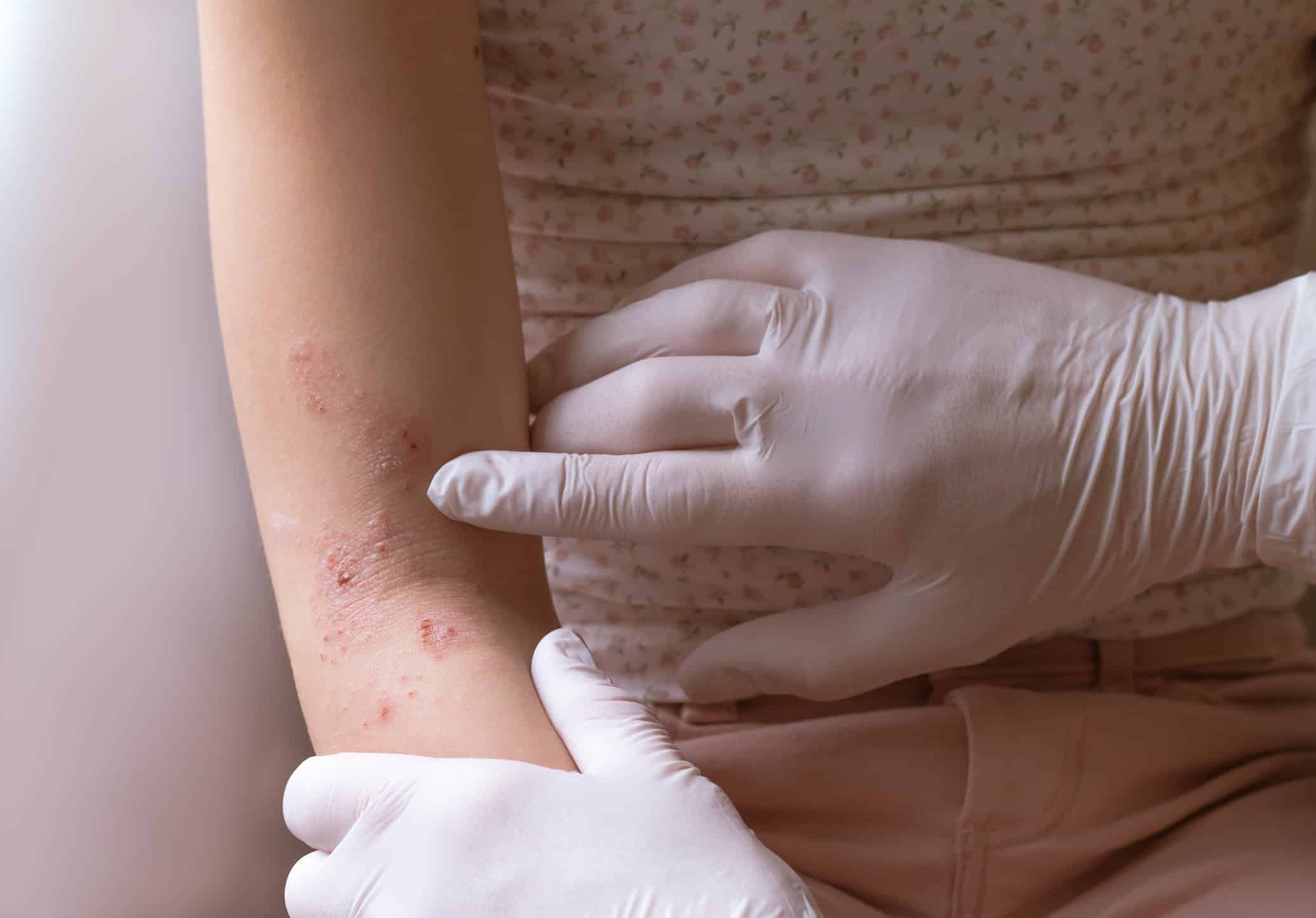
Microbial risk factors for atopic dermatitis, especially identical S. aureus strains, could be transferred by close physical contact in the home.
Patients with moderate to severe atopic dermatitis (AD) experience complications like frequent flares, superimposed bacterial and viral skin infections, and significantly impaired QOL from intense itching and sleep deprivation, explains Elizabeth Tham, MBBS, MRCPCH.
“Skin colonization with Staphylococcus aureus (S. aureus) has been shown to be linked with persistent severe disease and other complications, such as food allergy,” Dr. Tham says. “Eradication of S. aureus may improve disease severity but is currently only available through antiseptic techniques, such as bleach baths, antiseptic washes, and antibiotics. Symptom relief is often temporary, and recolonization is common. Taking lessons learned from the field of infectious diseases, it was thus postulated that skin microbiome sharing among individuals in close contact, such as parents/caregivers of children with AD, may serve as reservoirs of microbes, leading to recolonization. This gap is important to address, as current therapeutic approaches for AD are solely focused on the patient and not their household/close contacts.”
Microbes Could Be Transferred Through Physical Contact
For a study published in the Journal of Allergy and Clinical Immunology, Dr. Tham, Minghao Chia, PhD, and colleagues sought to better understand shared skin microbiomes between children with AD and their adult caregivers. The researchers recruited children aged 0-10 with moderate to severe AD, an equal number of healthy age-matched controls, and one healthy adult primary caregiver per child. Skin tape strips and skin swabs were collected from all participants according to established protocols. Skin microbial DNA and S. aureus strains isolated from the skin of participants were sequenced to uncover common bacterial signatures between primary caregiver and child in AD households versus controls.
“Our findings suggest that microbial risk factors for AD, especially identical S. aureus strains, could be transferred by close physical contact in the home, and that the skin of healthy caregivers may be a reservoir for microbial sharing,” Dr. Tham says. “Our research provides a basis for larger scale investigations into intra-familial transmission of pathogenic S. aureus strains and the overall skin microbiome community in AD. Evidence of skin microbial sharing between parent and patient further supports the inclusion of healthy caregivers in strategies for treating recurrent pediatric AD.”
Skin Microbiome Not a Static Entity, But a Dynamic Community
The study team observed distinctive microbial signatures in the non-lesional skin of children with AD and their healthy caregivers, according to Dr. Chia. “A higher ratio of S. aureus abundances relative to the commensal Staphylococcus hominis (S. hominis; the A/H ratio) was found to be a sensitive and specific marker for affiliation to an AD household, regardless of whether they were otherwise healthy adults or children,” he says. “The altered balance between S. aureus bioburden, relative to S. hominis on the skin of caregivers, drives home the point that there is sharing of skin microbes relevant to AD between caregivers and their children, and that S. aureus and S. hominis may antagonize each other (Figure).”
Dr. Tham concludes that this research highlights the skin microbiome not being a static entity but a dynamic community that can be shared, or perhaps even transferred, between individuals in close contact. “This is a novel concept previously understudied in the field of AD,” she notes. “It implies that therapeutic approaches aimed at eradication of pathogenic microbes like S. aureus from AD skin may require extension of treatment to those in close contact with them to achieve sustained microbial control.”
Valuable Adjunctive Therapies May Optimize AD Control
The researchers emphasize that such approaches would not stand alone but may be valuable adjunctive therapies to optimize AD control in selected patients, such as those with persistent S. aureus colonization or recurrent skin infections.
“Further research is required to uncover directionality of skin microbial transfer between individuals in close contact,” Dr. Tham adds. “It can also help identify optimal approaches to achieve sustained household microbial decolonization as a potential adjunct to conventional AD therapy and to identify key subgroups of patients with AD who would benefit from such a therapeutic approach.”