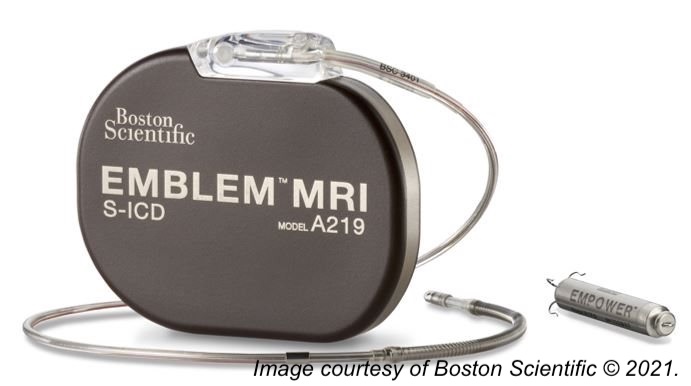
Surgeons at the Cleveland Clinic have successfully implanted the first leadless pacemaker combined with a defibrillator system in two patients as part of the ongoing, global MODUAR ATP clinical trial. The new device—the first of its kind—is a combination of a leadless pacemaker and subcutaneous implantable cardioverter defibrillator and was designed to deliver treatment for patients with both low and high heart rates.
Thanks to new technology, this leadless system is a combination of the EMPOWER Modular Pacing System and the EMBLEM Subcutaneous ICD (Boston Scientific), and as such, is not susceptible to the problems that conventional pacemaker ICDs are subject to–including increased risks of thrombosis and lead deterioration over time.
Daniel Cantillon, MD, research director and associate section head of Cardiac Electrophysiology and Pacing in the Heart, Vascular & Thoracic Institute, Cleveland Clinic, and global principal investigator of the MODULAR ATP trial, told BreakingMED: “For the first time, a leadless pacemaker defibrillator system allows us to treat sudden cardiac arrest without the need for indwelling transvenous leads (wires) threaded into the blood vessels, which cause many unwanted complications over time. This new system provides pacing stimulation to painlessly disrupt both lethal fast rhythms like ventricular tachycardia, and slow rhythms like asystole while retaining the shock capability of the ICD as a ’last line of defense.’”
Leadless pacemakers provide only single-chamber ventricular pacing and lack defibrillation capacity and are suitable for patients with permanent atrial fibrillation with bradycardia or bradycardia-tachycardia syndrome, or those who do not frequently require pacing.
By combining the leadless pacemaker with a defibrillator system, the Cleveland Clinic hopes to extend use to a larger patient population.
Cantillon explained the benefits of this new investigational device.
“Traditional pacemaker-defibrillators have leads (wires) threaded through the blood vessels entering into the heart, which are vulnerable to complications affecting approximately 1 in 6 patients by 3 years, including dislodgements, blood clots (venous occlusive disease) and other system complications. This system eliminates those leads (wires) while retaining the ability to provide pacing support to our patients. It can disrupt potentially lethal arrhythmias like ventricular tachycardia by painlessly terminating them while withholding the shock therapy of the ICD itself as a last resort. While life-saving, ICD shocks are painful and psychologically traumatizing to our patients,” he told BreakingMED.
“The S-ICD (subcutaneous ICD) first detects a lethal arrhythmia like ventricular tachycardia that occurs spontaneously in patients with congestive heart failure. Think of a medical drama on TV where it’s ’code blue’ with squiggly lines on the monitor. With this system, what the ICD does is analyze the rhythm while charging its battery and getting ready to deliver a life-saving shock. In those crucial seconds, it sends a coded message through the patient’s body to the leadless pacemaker, which receives it and delivers a sequence of pacing stimulation—that we program ahead of time—in order to attempt to painlessly disrupt the arrhythmia. Prior studies suggest this can be successfully in up to 75% cases of monomorphic ventricular tachycardia, which is by far the most common lethal arrhythmia. If it succeeds, then the ICD does not deliver the shock. If it fails, then the ICD immediately delivers the rescue shock. All of this happens within seconds, as death can occur within minutes. We actually test that capability at the time of implant in the operating room in a highly controlled environment to ensure that it works,” he added.
Cantillon and fellow researchers of the multi-center, nonrandomized MODULAR ATP trial plan to enroll 300 patients who require a new ICD or who already have an implanted subcutaneous-ICD system across 50 centers in the U.S., Canada, and Europe. Results are anticipated in 12-24 months.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
Cantillon is a consultant for Boston Scientific.
Cat ID: 914
Topic ID: 74,914,730,914,192,925