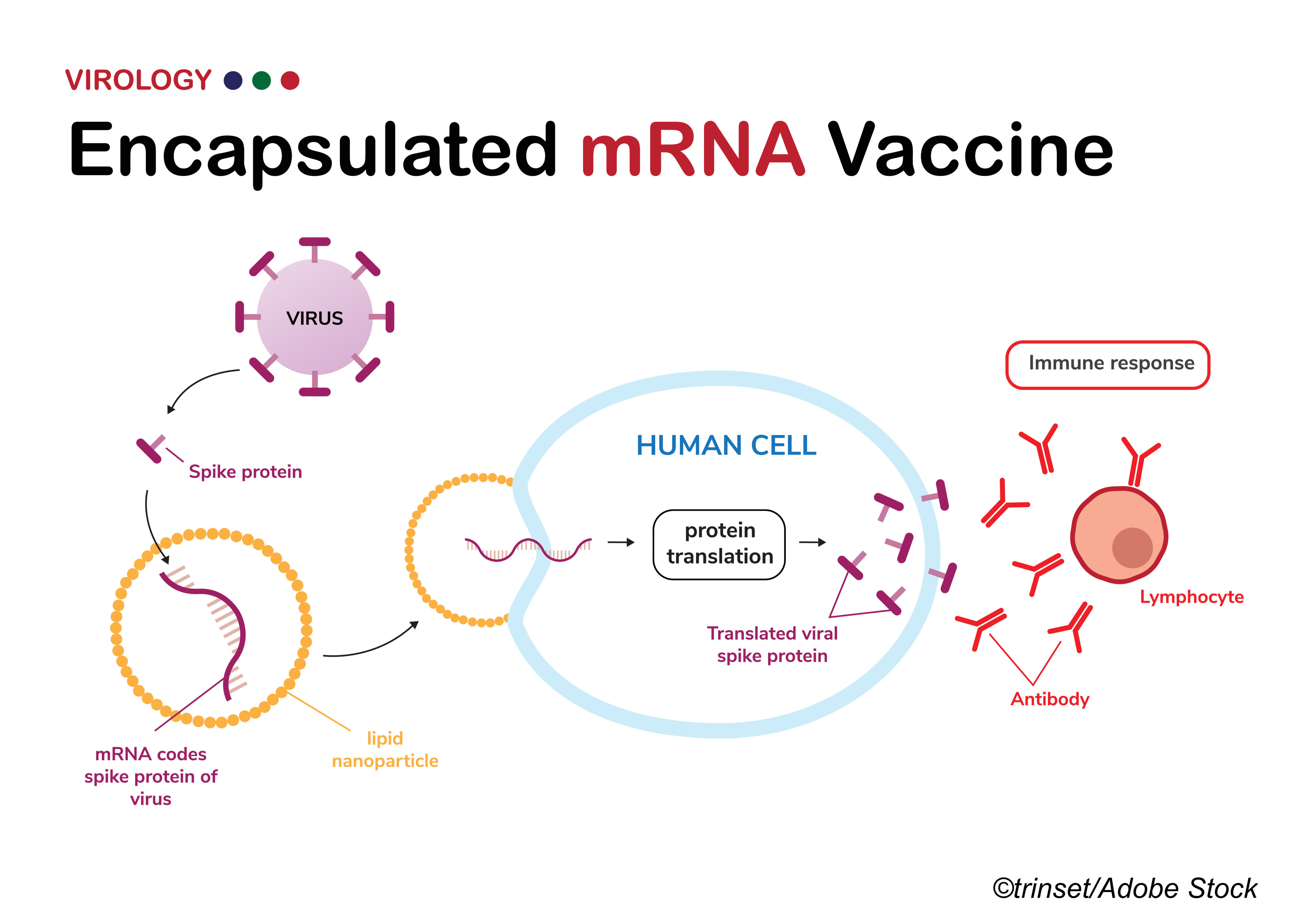
Final analysis of data from a pivotal mRNA vaccine phase III trial showed continued high efficacy against illness and severe Covid-19 more than 5 months after full vaccination.
Initial findings from the COVE study showed the mRNA-1273 vaccine (Moderna) to have 94% efficacy against Covid-19 illness, with an acceptable safety and side-effect profile 64 days after full vaccination.
In the updated analysis of the blinded phase of the trial involving roughly 30,000 participants followed for a median of 5.3 months after receiving a second dose, the vaccine remained 93% effective for preventing Covid-19 illness and 98% effective against severe disease.
And subgroup analysis showed consistent vaccine efficacy across all included racial, ethnic, and age groups, and among study participants with health conditions including chronic lung disease, cardiac disease, severe obesity, diabetes, and liver disease.
In another vaccine trial update, the mRNA-1273 and BNT162b2 (Pfizer-BioNTech) vaccines were both found to be highly effective under real-world conditions in health-care workers for preventing symptomatic Covid-19 and severe illness, including those most at risk for severe Covid-19 due to pre-existing conditions or significant exposure to patients with Covid-19 and those belonging to racial groups disproportionately affected by the pandemic.
Both studies were published online Sept. 22 in The New England Journal of Medicine.
The COVE Study analysis “enrolled 30,415 participants, including 15,209 assigned to receive the Moderna mRNA vaccine, and 15,206 assigned to receive placebo under blinded randomization… More than 96% of participants received both injections, 2.3% had evidence of SARS-CoV-2 infection at baseline, and the median follow-up was 5.3 months in the blinded phase of the trial,” the study authors wrote.
Among the main findings from the follow-up analysis, vaccine efficacy was:
- 93.2% (95% CI, 91.0- 94.8) for preventing Covid-19, with 55 confirmed cases in the mRNA vaccine group (9.6 per 1000 person-years; 95% CI, 7.2-12.5) and 744 in the placebo group (136.6 per 1000 person-years; 95% CI, 127.0-146.8).
- 98.2% (95% CI, 92.8-99.6) for preventing severe disease, with 2 cases in the mRNA-1273 group and 106 in the placebo group.
- 63.0% (95% CI, 56.6-68.5) for preventing asymptomatic infection starting 14 days after the second injection, with 214 cases in the mRNA-1273 group and 498 in the placebo group.
“No safety concerns were identified in this trial. However, robust safety surveillance systems have identified rare events during the global distribution of Covid-19 vaccines that the phase 3 studies were not powered to detect; thus, continued vigilance is warranted, including monitoring for anaphylactic reactions, especially in persons with allergic phenotypes, and for other potential unexpected reactions, such as myocarditis in adolescents and young adults,” the researchers wrote.
The health care worker trial, funded by the CDC, was a test-negative, case-control study involving health care workers in 25 U.S. states.
“Cases were defined on the basis of a positive polymerase chain-reaction (PCR) or antigen-based test for SARS-CoV-2 and at least one Covid-19–like symptom,” the study authors wrote. “Controls were defined on the basis of a negative PCR test for SARS-CoV-2, regardless of symptoms, and were matched to cases according to the week of the test date and site.”
Conditional logistic regression with adjustment for age, race and ethnic group, underlying conditions, and exposures to workers with Covid-19, was used to estimate the efficacy of partial vaccination (assessed 14 days after the first dose through 6 days after the second dose); complete vaccination was assessed ≥7 days after the second dose was given.
Among the 1,482 cases and 3,449 controls, vaccine efficacy was:
- 77.6% for partial vaccination (95% CI, 70.9-82.7) with BNT162b2 and 88.9% (95% CI, 78.7-94.2) with mRNA-1273.
- 88.8% (95% CI, 84.6- 91.8) and 96.3% (95% CI, 91.3-98.4), following complete vaccination, respectively for the two vaccines.
- Similar in subgroups defined according to age (<50 years or ≥50 years), race and ethnic group, presence of underlying conditions, and level of patient contact.
“In this population of health care personnel, vaccine effectiveness was similar among persons with underlying medical conditions or other risk factors for severe Covid-19, including pregnancy; in different subgroups of health care personnel defined according to job category; and in racial and ethnic groups that have been disproportionately affected by the pandemic,” the researchers wrote.
“The long-term duration of protection and the effectiveness of these vaccines against emerging variants is unknown and should be monitored to indicate whether changes to vaccine composition or vaccine policy are needed.”
-
In updated analysis of the COVE study, the mRNA-1273 vaccine remained 93% effective for preventing Covid-19 illness and 98% effective against severe disease a median of 5.3 months after full vaccination.
-
In another trial, both the mRNA-1273 and BNT162b2 vaccines were found to be highly effective under real-world conditions in health-care workers for preventing symptomatic Covid-19 and severe illness.
Salynn Boyles, Contributing Writer, BreakingMED™
The COVE study was funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases. The study be Pilishvili et al. was funded by the Centers for Disease Control and Prevention.
Cat ID: 190
Topic ID: 79,190,730,933,190,926,192,927,151,928,925,934