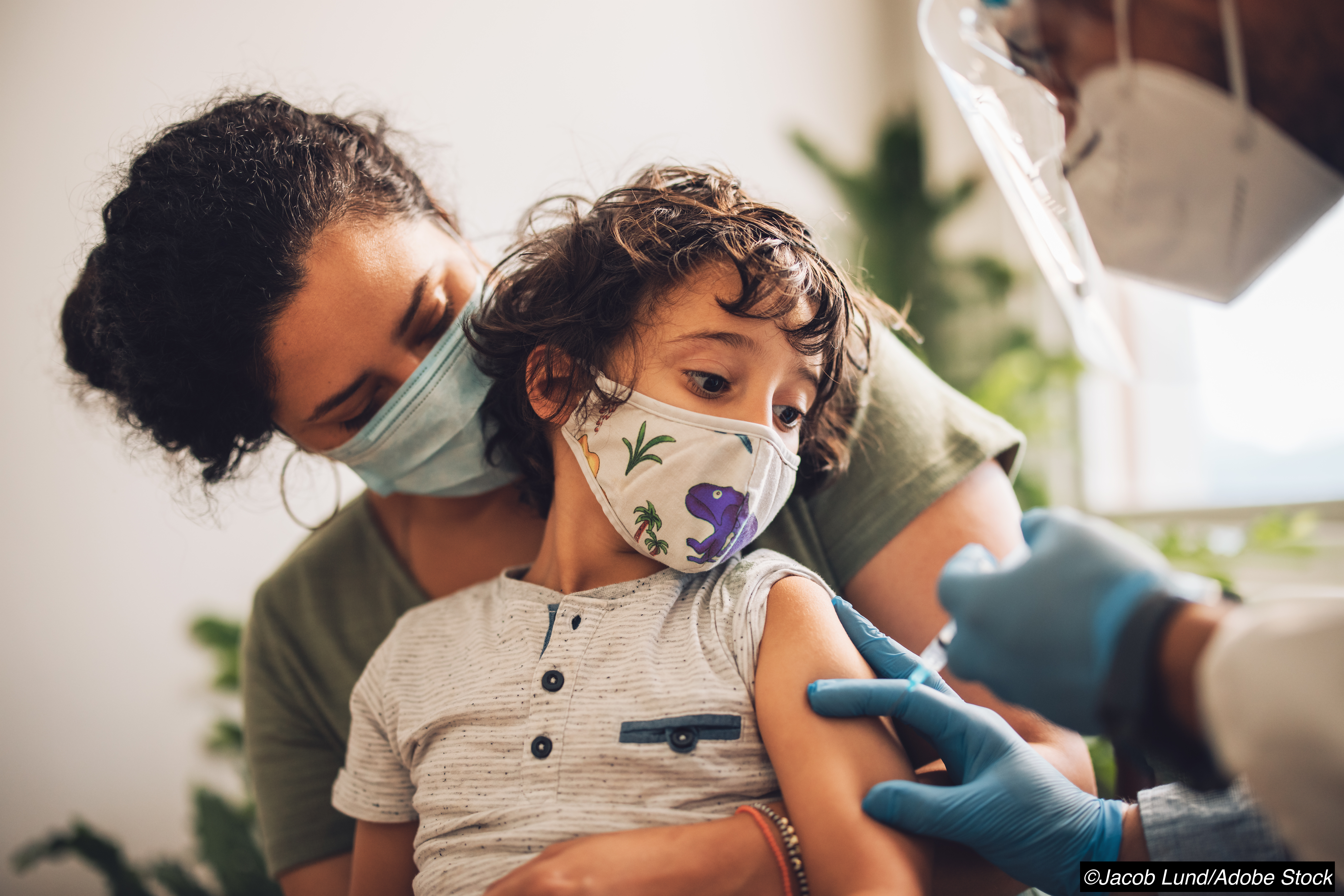
Peer-reviewed findings from the clinical trial that led to the approval of the BNT162b2 Covid-19 vaccine for use in children 5 to 11 years of age show kid-sized doses of the vaccine to be more than 90% effective for preventing infection in the age group.
The vaccination regimen—consisting of two 10-μg doses of the BNT162b2 mRNA vaccine given 21 days apart—was associated with an efficacy of 90.7% (95% CI, 67.7-98.3) against infection on assessment seven days or more after the second dose was administered.
Data from the ongoing trial, published Tuesday in The New England Journal of Medicine, included early findings on 2,268 children between the ages of 5 and 11 years who received two doses of the vaccine or placebo.
Just over a week ago, the U.S. Food and Drug Administration authorized the BNT162b2 vaccine at the 10 microgram dose for use in children as young as 5 years of age, noting that children between the ages of 5 and 11 years accounted for almost 40% of recent Covid-19 cases among non-adults in the U.S.
The vaccine has been approved since early May for use in children age 12 to 15 years at the 30 μg-doses approved for adults.
The emergence of the SARS-CoV-2 Delta variant led to a surge of Covid-19 cases among children beginning last summer, with the CDC reporting a 5-fold increase in hospitalizations due to the virus among children and adolescents during a six-week period between late-June to mid-August.
Between March of 2020 and August of 2021, 562 children in the U.S. were hospitalized with Covid-19, according to the CDC, with 68% of these admissions occurring among Black or Hispanic children and 1-in-3 (32%) hospitalization involving children with no underlying medical conditions.
A total of 94 Covid-19-related deaths had been reported to the CDC among children between the ages of 5 and 11 years through mid-October.
The phase I, Pfizer-BioNTech-sponsored dose-finding study and an ongoing phase II/III randomized trial were designed to investigate the safety, immunogenicity, and efficacy of two doses of the BNT162b2 vaccine administered 21 days apart in children 6 months to 11 years of age. Data on children under 5 years of age have not yet been published.
The dosage level of 10 μg was selected for ongoing trials based on reactogenicity findings from the phase I analysis.
In the phase II/III trial, the children were randomly assigned to receive the BNT162b2 vaccine (1,517 children) or placebo (751 children).
“Immune responses one month after the second dose of BNT162b2 were immunologically bridged to those in 16-to-25-year-olds from the pivotal trial of two 30-μg doses of BNT162b2,” wrote researcher Emmanuel Walter, MD, of the Duke Human Vaccine Institute, Durham, North Carolina, and colleagues.
They noted that no serious vaccine-related adverse events were reported among the study participants.
A month after receiving the second dose, the geometric mean ratio of SARS-CoV-2 neutralizing titers in 5-to-11-year-olds to those in 16-to-25-year-olds was 1.04 (95% CI, 0.93-1.18).
This ratio met “the prespecified immunogenicity success criterion (lower bound of two-sided 95% CI, >0.67; geometric mean ratio point estimate, ≥0.8),” the researchers wrote, adding that Covid-19 with onset seven days or more after the second dose was reported in three recipients of the BNT162b2 vaccine and in 16 placebo recipients (vaccine efficacy, 90.7%; 95% CI, 67.7-98.3).
Study limitations included the absence of longer-term follow-up data on the children, who will be followed for two years.
“This study was also not powered to detect potential rare side effects of BNT162b2 in 5-to-11-year-olds. However, the safety of BNT162b2 observed in the study combined with widespread use of BNT162b2 in older populations should provide reassurance,” the researchers wrote.
“Moreover, an expanded cohort of 5-to-11-year-olds is being assessed in the present study, and additional safety assessments are in progress. Further limitations are that concomitant administration of BNT162b2 with other vaccines was not assessed, and cell-mediated responses to immunization are not yet available.”
-
Peer-reviewed findings from the clinical trial that led to the approval of the BNT162b2 Covid-19 vaccine for use in children 5 to 11 years of age show the vaccine to be more than 90% effective for preventing infection in the age group.
-
The BNT162b2 vaccine was authorized by the FDA on Oct. 29 at a dosage of 10 micrograms for use in children 5 to 11 years of age.
Salynn Boyles, Contributing Writer, BreakingMED™
This research was funded by BioNTech and Pfizer. Walter reported receiving grants from Moderna, Pfizer and Vaxcyte. Researcher Wuilliam Gruber reported being an employee of Pfizer and holding stock and stock options in the company. Corresponding researcher Alejandra Gurtman also reported being a Pfizer employee.
Cat ID: 190
Topic ID: 79,190,730,933,190,926,138,44,192,561,927,151,928,925,934