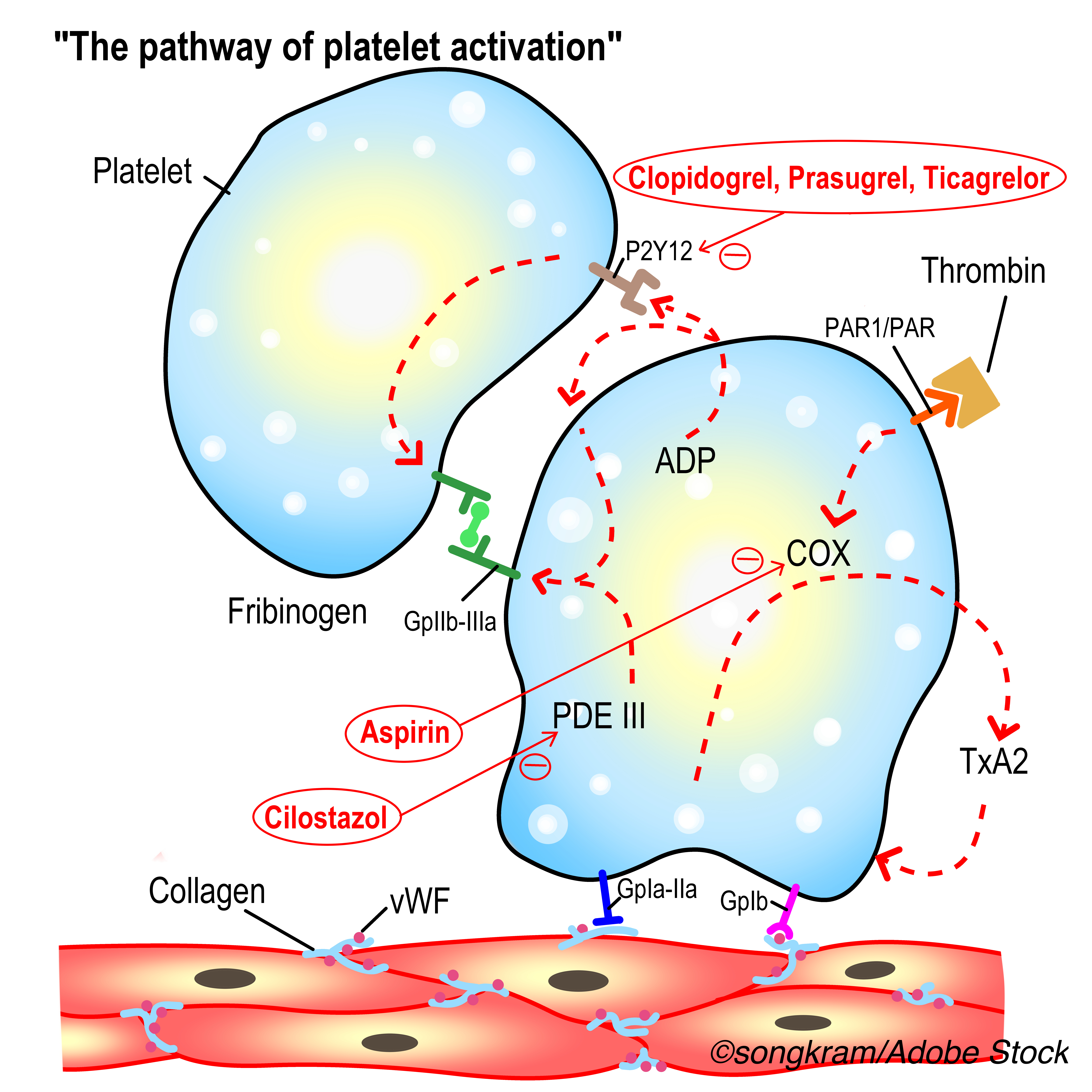
In patients with stable coronary artery disease (CAD) and type 2 diabetes without prior myocardial infarction (MI) or stroke, dual antiplatelet therapy with ticagrelor plus aspirin reduced the risk of ischemic events regardless of the duration of diabetes, baseline HbA1c, or antihyperglycemic therapy.
Treatment did, however, increase the risk of bleeding, according to a post-hoc analysis of the THEMIS and THEMIS-PCI studies published in the Journal of the American College of Cardiology.
“In brief, THEMIS (n=19,220) and the pre-specified THEMIS-PCI (Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study-Percutaneous Coronary Intervention) (n=11,154) subgroup analysis suggested that dual antiplatelet therapy (DAPT) with ticagrelor and low-dose aspirin may specifically benefit individuals with type 2 diabetes mellitus and stable atherosclerosis but without a history of MI or stroke and who had previously undergone a PCI,” wrote Lawrence A. Leiter, MD, of Li Ka Shing Knowledge Institute of St. Michael’s Hospital and the University of Toronto, Toronto, Ontario.
“The U.S. Food and Drug Administration has since, based on THEMIS, additionally approved ticagrelor to reduce the risk of a first MI or stroke in patients with coronary artery disease who are at high risk for such events regardless of whether they have diabetes or not, and Health Canada, based on THEMIS-PCI, has expanded approval of ticagrelor more specifically in patients with coronary artery disease, type 2 diabetes, and a history of PCI,” they added.
Leiter and colleagues conducted these post-hoc analyses to assess whether the primary efficacy outcome in the original THEMIS studies (cardiovascular death, MI, stroke: 3-point major adverse cardiovascular events [MACE]), the primary safety outcome (major bleeding) and net clinical benefit differed with diabetes-related factors, which included patients’ diabetes duration, HbA1c, and antihyperglycemic treatments.
Researchers of the original THEMIS and THEMIS-PCI studies included 19,220 patients (31.4% women) with CAD confirmed by angiography and type 2 diabetes but no history of MI or stroke who were randomized to treatment with either ticagrelor (90 mg twice daily initially, changed to 60 mg twice daily after one year) or placebo plus low-dose aspirin (75-150 mg) for a median follow-up of 39.9 months.
Upon analyses, Leiter and fellow researchers found that the incidence of 3-point MACE was increased with diabetes duration (6.7% for 5 years or greater, 11.1% for greater than 20 years of diabetes), and HbA1c (6.4% for less than or equal to 6.0%, 11.8% for greater than 10.0%). Similar results were seen in THEMIS-PCI.
Across subgroups divided according to duration of diabetes and HbA1c, the benefits of ticagrelor plus aspirin on 3-point MACE reduction was consistent in THEMIS (HR: 0.90; 95% CI: 0.81-0.99; P=0.04), and in THEMIS-PCI (HR: 0.85; 95% CI: 0.74-0.97; P=0.013).
The major bleeding—as defined by Thrombolysis in Myocardial Infarction—was 1.6% in the overall THEMIS population (HR: 2.32; 95% CI: 1.82 to 2.94; for ticagrelor vs placebo; P<0.001) and 2.0% in the THEMIS-PCI subcohort (HR: 2.03; 95% CI: 1.48-2.76; for ticagrelor vs placebo; P<0.0001), and did not differ by diabetes duration or HbA1c. The major bleeding event rate was similarly increased by treatment with ticagrelor across all subgroups (HR: 2.32; P˂0.001).
In THEMIS, patients treated with insulin had a higher risk for 3-point MACE (HR: 1.65; 95% CI: 1.49-1.83; P<0.0001), but these risks were similar to patients not taking insulin (HR: 0.92; 95% CI: 0.71-1.19; P=0.520).
In comparisons of patients treated with sulfonylurea and those who were not, risks for 3-point MACE (HR: 1.00; 95% CI: 0.91-1.11; P=0.92) and TIMI major bleeding (HR: 1.12; 95% CI: 0.89-1.41; P=0.34) did not differ. No risk differences for 3-point MACE or bleeding risk were seen when patients were analyzed according to treatment/nontreatment with metformin, DPP-4 inhibitors, SGLT2 inhibitors, or GLP-1 receptor agonists.
The net clinical benefit of treatment, which was a composite of all-cause mortality, MI, stroke, fatal bleeding, or intracranial bleeding in the intention-to-treat population, was similar in both THEMIS and THEMIS-PCI across diabetes duration and HbA1c.
“Notably, ticagrelor was generally associated with greater net clinical benefit across the diabetes duration and HbA1c spectra in the THEMIS-PCI subcohort but not in the overall THEMIS cohort,” noted Leiter et al.
“The present results indicate that together, ticagrelor plus aspirin reduced the incidence of MACE regardless of baseline duration of diabetes and baseline HbA1c but at the expense of major bleeding events. Notably, the combination of ticagrelor plus aspirin resulted in generally consistent and favorable net clinical benefit across the various diabetes-related factors in THEMIS-PCI, but not in the overall THEMIS population,” they concluded.
In an accompanying editorial, Jean-Guillaume Dillinger, MD, PhD, and Patrick Henry, Md, PhD, both of the Université de Paris, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, France, wrote:
“The authors highlight the influence of 2 main diabetes-related factors (i.e., DM duration and HbA1c level) on the rate of 3-point MACE in the THEMIS trial. Due to the narrow benefit-risk balance of ticagrelor plus aspirin in DM patients with chronic CAD, the results of this study may allow clinicians to identify high-risk DM patients.”
They added: “However, identification of high-risk DM patients does not necessarily imply greater benefits of ticagrelor, which makes the equation complex. Notably, in the study by Leiter et al. despite the higher rate of 3-point MACE in the high-risk subgroups (prolonged DM duration, high HbA1c level), the absolute risk reduction by ticagrelor was not significantly different regardless of the ischemic event rates of the subgroups. Nevertheless, clinicians should consider the combination of ticagrelor and aspirin as a new treatment option to achieve net clinical benefit for high-risk DM patients.”
Dillinger and Henry stressed that although ticagrelor and aspirin may be a new option for a select group of patients with diabetes, treatment should be preceded by careful assessment of bleeding risks, duration of diabetes, HbA1c level, and prior PCI.
Study limitations included the use of baseline HbA1c and antihyperglycemic regimens, which may have changed during the study period; low rates of novel antihyperglycemic agents; and the restrictive definition of net clinical benefit.
-
Combination treatment with ticagrelor and aspirin is an effective option for selected patients with diabetes with a high cardiovascular risk.
-
Ticagrelor plus aspirin together reduced the incidence of MACE, regardless of diabetes duration or baseline HbA1c levels, but increased major bleeding events.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
The THEMIS study was funded and sponsored by AstraZeneca Research & Development. The statistical analyses were conducted independently from but with funding from AstraZeneca.
Leiter has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS, Janssen, Novartis, Novo Nordisk, and Sanofi; has received grants from Esperion, GSK, Kowa, Lexicon, Novartis, and The Medicines Company; and has received personal fees from Merck and Servier.
Dillinger has received consulting and lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Sanofi, and Daiichi-Sankyo; and has received grants from Bayer, Bristol Myers Squibb/Pfizer, and Biosensors.
Henry has received consulting and lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Sanofi, and Daiichi-Sankyo; and has received grants from Bayer.
Cat ID: 308
Topic ID: 74,308,730,308,914,12,13,669,918