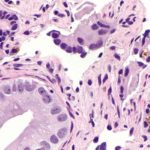
1. Patients with hormone-sensitive prostate cancer receiving darolutamide, an androgen-receptor inhibitor, in addition to standard therapy demonstrated longer overall survival than patients receiving only standard therapy of androgen-deprivation therapy and docetaxel.
Evidence Rating Level: 1 (Excellent)
Study Rundown: A subset of prostate cancers are hormone-sensitive, meaning that they are dependent on androgen-receptor activity and may respond to inhibitors of this pathway. Darolutamide is a potent androgen-receptor inhibitor with demonstrated antitumor activity on its own in the phase 3 clinical trial. The objective of this study was to investigate the activity of darolutamide in combination with standard therapy of androgen-deprivation therapy and docetaxel. In this phase 3 trial, patients with hormone-sensitive prostate cancer were randomized in a 1:1 ratio to receive oral darolutamide or placebo, in addition to standard therapy. With respect to its primary endpoint, the study found that darolutamide significantly improved overall survival compared to the placebo. Secondary endpoint measures including time to pain progression, symptomatic skeletal event-free survival, and time to first symptomatic skeletal event were also improved with darolutamide therapy. Rates of adverse events were similar between groups with neutropenia being the most common severe adverse event. Taken altogether, the study supports the addition of darolutamide to standard therapy regimens for hormone-sensitive prostate cancer. The limitations of this study include limited generalization given that all enrolled patients had good performance status and had metastatic disease. Thus, the benefit-risk is unknown for those with less advanced disease.
Click to read the study in NEJM
Relevant Reading: Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide
In-Depth [randomized controlled trial]: In this international, phase 3 randomized control trial, 1306 patients with histologically diagnosed metastatic cancer, good performance status, and deemed to be hormone-sensitive were enrolled. Patients were randomized in a 1:1 ratio to either receive 600mg oral darolutamide or placebo in addition to the standard therapy regimen of androgen-deprivation therapy and docetaxel. Patients were assessed every 12 months for measures of survival, symptom progression, initiation of therapy, and adverse events. The primary endpoint was overall survival at the data cutoff date, analyzed by stratified log-rank test using Kaplan-Meier survival curves or hazard ratios using the Cox proportional-hazards model. Secondary endpoints assessed measures of pain progression, skeletal symptom development related, initiation of additional antineoplastic therapy or opioid use, and safety. The study found that patients receiving additional darolutamide treatment had a significantly better 4-year overall survival rate at 62.7% compared to patients only receiving standard therapy at 50.4%. This translates into a 32.5% lower risk of death in the darloutamide group (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.57-0.80; P<0.001). Patients receiving darolutamide also showed improvement in secondary endpoints including increased time to development of castration-resistant disease, increased time to pain progression, increased time to first symptomatic skeletal event, and increased time to systemic anti-neoplastic therapy. Rates of serious adverse events were similar between the two groups (44.8% in darolutamide groups vs. 42.3% in placebo group), with alopecia and neutropenia being the most commonly reported side effects. Overall, the results of this phase 3 clinical trial were able to support the addition of darolutamide, a potent androgen-receptor inhibitor, to standard therapy in patients with hormone-sensitive prostate cancer without increasing serious adverse events.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.