WASHINGTON — The FDA approved a new indication for mirabegron (Myrbetriq) to treat patients three years and older with neurogenic detrusor overactivity (NDO).
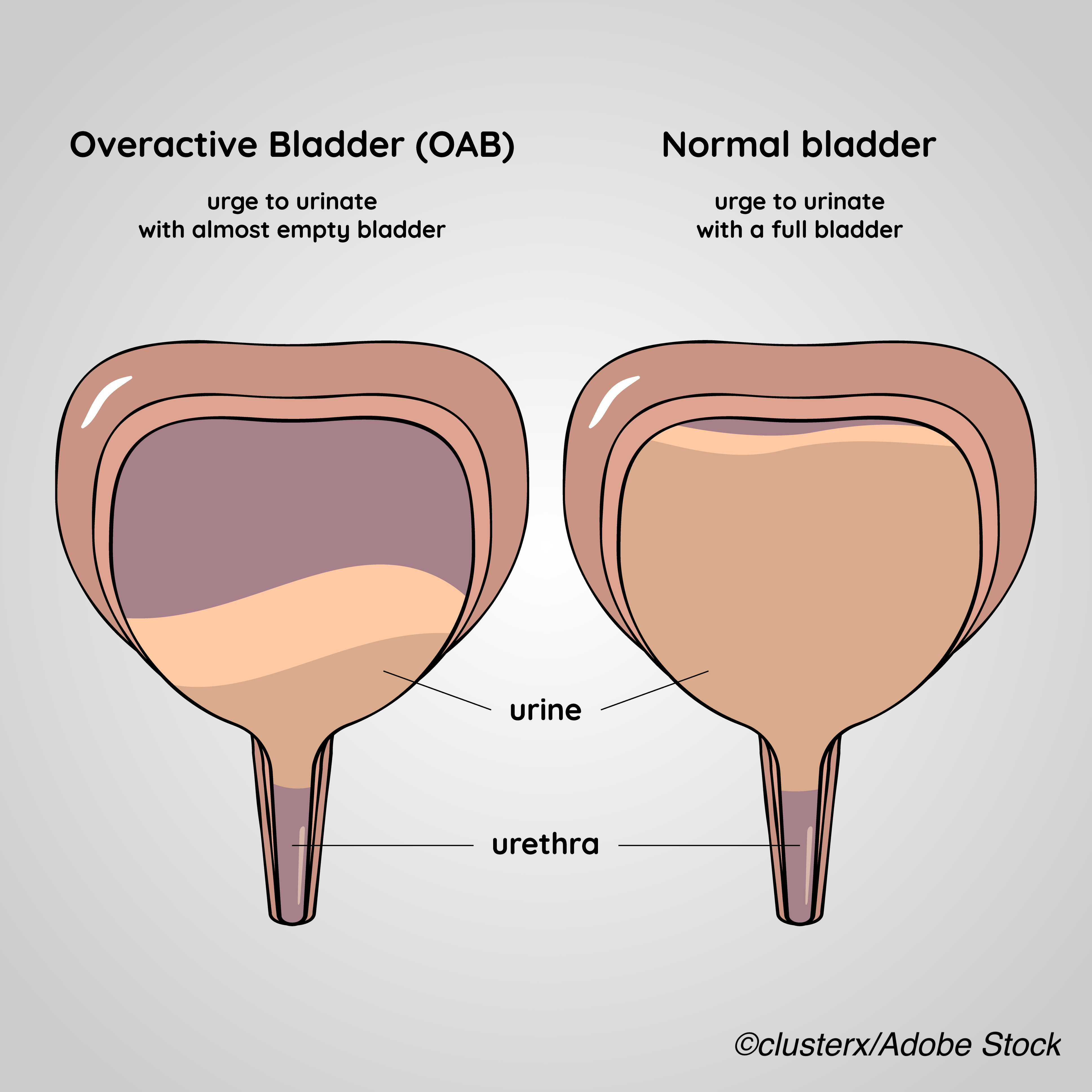
NDO, a bladder dysfunction that stems from neurological impairments caused by congenital conditions, such as spina bifida, or nervous system diseases or injuries, such as spinal cord injury, causes bladder wall muscle overactivity that results in sporadic bladder muscle contraction, increasing bladder pressure and decreasing the bladder’s capacity to hold urine. This increased bladder pressure can cause upper urinary tract damage — including permanent kidney damage — if left untreated, as well as urinary urgency, frequency, and incontinence.
Mirabegron, which was previously approved to treat overactive bladder in adult patients, will be available to pediatric patients in two forms — extended-release oral tablets and extended-release oral suspension (Myrbetriq Granules).
“Today’s action is a positive step for the treatment of NDO in young patients,” Christine P. Nguyen, MD, director, FDA’s Division of Urology, Obstetrics and Gynecology, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, Center for Drug Evaluation and Research, said in a statement. “Mirabegron… works by a different mechanism of action from the currently approved treatments, providing a new treatment option for these young patients. We remain committed to facilitating the development and approval of safe and effective therapies for pediatric NDO patients.”
The FDA based its approval on results from a study assessing mirabegron tablets and oral suspension among a cohort of 86 patients ages 3-17. According to the FDA, improvements in patients’ maximum cystometric capacity, number of detrusor contractions, volume of urine held until first detrusor contraction, and number of daily urine leakage episodes after 24 weeks of treatment.
The most common reported adverse events associated with mirabegron tablets and oral suspension for NDO were urinary tract infection, nasopharyngitis, constipation, and headache. The drug may also cause increased blood pressure and angioedema, the FDA warned.
Mirabegron tablets and oral-suspension “are two distinct products,” the agency added, “and they are not substitutable on a milligram-per-milligram basis.”
Mirabegron is manufactured by Astellas Pharma US, Inc.
John McKenna, Associate Editor, BreakingMED™
Cat ID: 138
Topic ID: 85,138,730,190,130,138,192,725,925