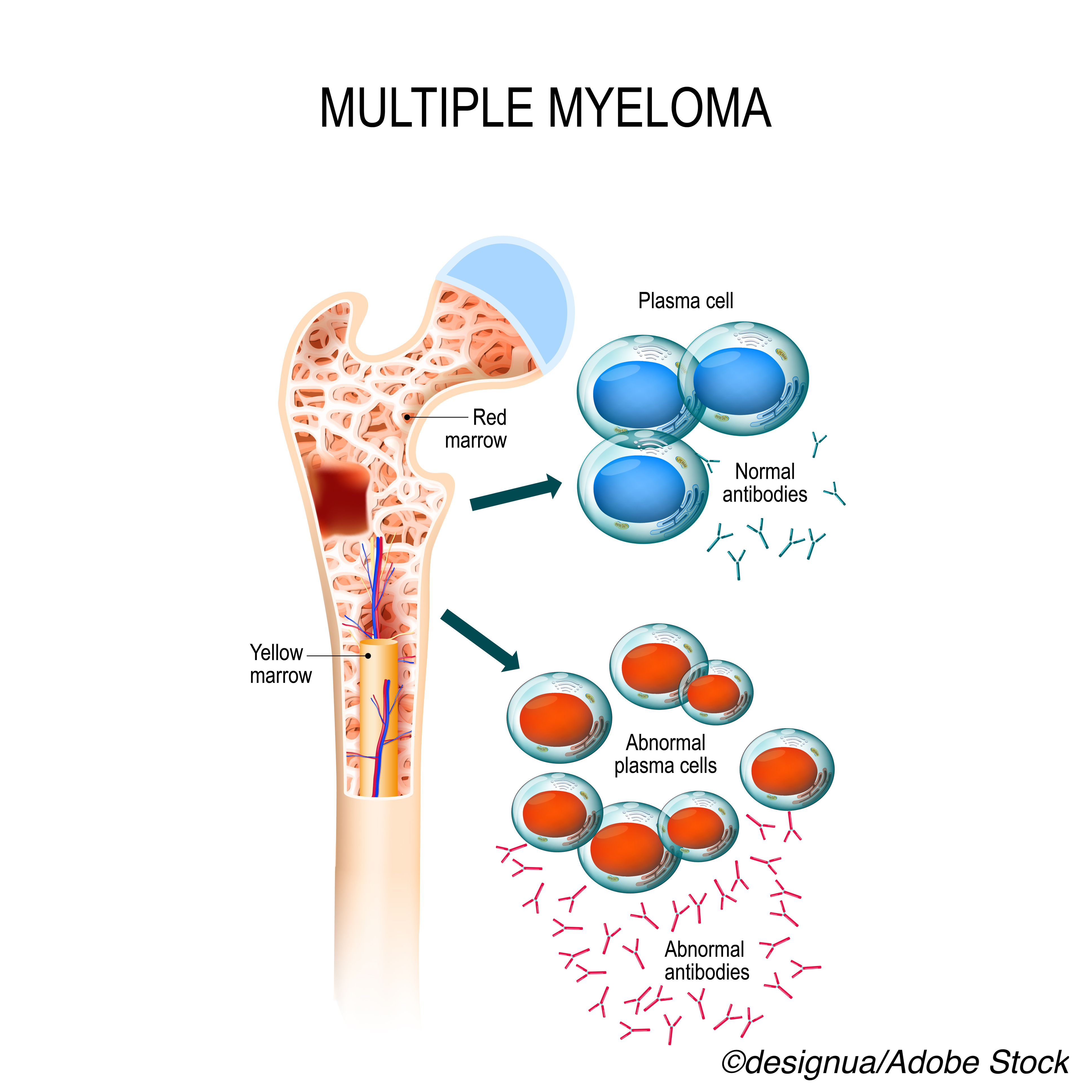
Melphalan flufenamide, otherwise known as melflufen, is the first anti-cancer peptide-drug conjugate to gain FDA-approval for multiple myeloma, the drug’s manufacturer, Oncopeptides AB, explained in a company press release. With this approval, the conjugate will be available for patients whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one CD38-directed monoclonal antibody.
The FDA gave melflufen an accelerated approval designation based on results from the single-arm, phase II HORIZON trial, which evaluated melflufen in combination with dexamethasone in 157 heavily pre-treated patients with relapsed or refractory multiple myeloma, including 119 participants who were triple-class refractory. Results of the HORIZON trial were published in the American Society of Clinical Oncology (ASCO)’s Journal of Clinical Oncology, as previously reported by BreakingMED.
The overall response rate among participants was 29%, and the response rate was 26% among the triple-class-refractory population, Paul Richardson, MD, of the R.J. Corman Professor of Medicine at Harvard Medical School/Dana-Farber Cancer Institute in Boston, and colleagues reported.
“In the all-treated population, median duration of response was 5.5 months, median progression-free survival was 4.2 months, and median overall survival was 11.6 months at a median follow-up of 14 months,” they wrote. “Grade ≥ 3 treatment-emergent adverse events occurred in 96% of patients, most commonly neutropenia (79%), thrombocytopenia (76%), and anemia (43%). Pneumonia (10%) was the most common grade 3/4 nonhematologic event. Thrombocytopenia and bleeding (both grade 3/4 but fully reversible) occurred concomitantly in four patients. GI events, reported in 97 patients (62%), were predominantly grade 1/2 (93%); none were grade 4.”
“While the treatment landscape for multiple myeloma has dramatically improved in recent years, once patients become resistant to existing classes of therapy they can face a very guarded prognosis,” Richardson said in the company’s press release. “Research has shown melphalan flufenamide to be a novel and innovative therapeutic option, which is active in refractory disease and has manageable toxicity, with the convenience of being administered by infusion once a month. Based on our findings, melphalan flufenamide is an important addition to the treatment armamentarium, with the potential to meaningfully improve outcomes in an area of important unmet medical need.”
The most common adverse events associated with melflufen included fatigue, nausea, diarrhea, pyrexia, and respiratory tract infection; the most common laboratory abnormalities included leukocytes decrease, platelets decrease, lymphocytes decrease, neutrophils decrease, hemoglobin decrease, and creatinine increase. The FDA prescribing information also includes warnings that the drug may cause thrombocytopenia, neutropenia, anemia, infections, secondary malignancies, and embryo-fetal toxicity — and giving melflufen at higher than the approved dose (20 mg) may lead to death, the agency added.
John McKenna, Associate Editor, BreakingMED™
Cat ID: 468
Topic ID: 78,468,730,468,192,925


