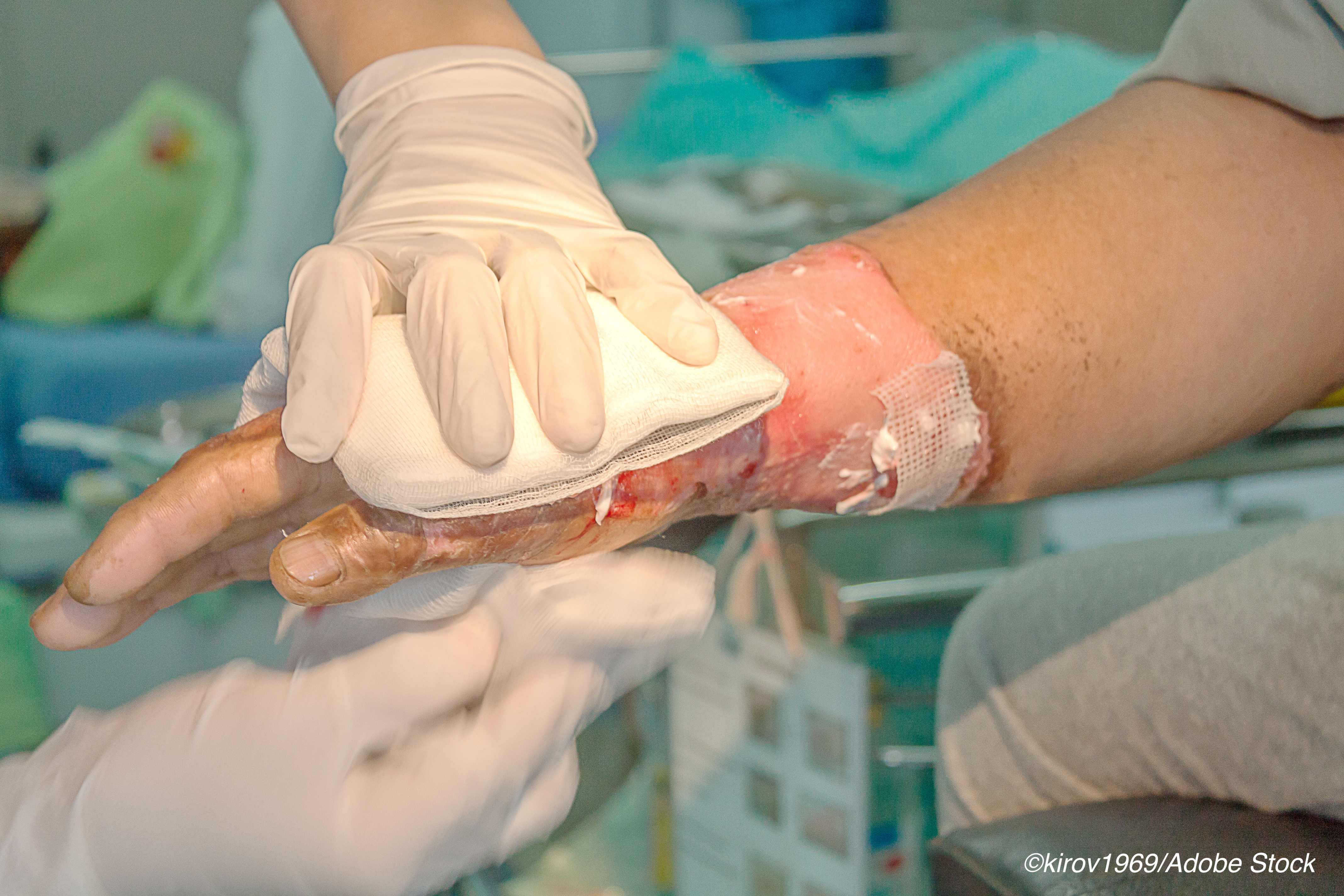
The FDA approved an engineered, bilayer topical skin tissue, StrataGraft, to treat adult patients with deep partial thickness thermal burns with remaining deep skin layers.
For many deep thermal burns, treatment involves an autograft, removing the damaged layers of skin and replacing it with healthy skin taken from other areas on the patient’s body. The StrataGraft—which is made with two kinds of human skin cells, keratinocytes and dermal fibroblasts, grown together to form a bi-layered cellularized scaffold—is designed to be applied to the burn area to decrease, or outright remove, the amount of health skin needed to produce an autograft.
“Serious burns can be an incredibly difficult injury to treat and can adversely affect more than just the skin. The goal of burn management is to help the patient return to the highest level of functionality and independence possible, while improving the overall quality of life,” said Peter Marks, MD, PhD, director of FDA’s Center for Biologics Evaluation and Research, in a statement. “This approval provides health care professionals a novel way to treat burn wounds.”
This approval was based on results from two randomized clinical studies that included a total of 101 adult patients with deep partial thickness thermal burns, the FDA explained.
“In both studies, two deep partial-thickness burn wounds of comparable area and depth on each patient were identified and randomized to receive either a single topical application of StrataGraft or autograft,” the agency wrote. “The effectiveness is demonstrated by the percentage of StrataGraft treatment sites that achieved a complete wound closure, and the significantly decreased need for autografts at the StrataGraft treatment sites.”
The FDA noted that, while mouse cells were used to produce human keratinocyte cells during initial stages of product development, they are no longer a part of the manufacturing process; nonetheless, StrataGraft is formally considered to be a xenotransplantation product, or a product involving tissues or cells from a different species.
“Due to StrataGraft containing cells from human donors and animal-derived materials, and because animal cells were used in early stages of product development, there is a risk of transmission of infectious diseases or agents although at this time transmission of infectious diseases or agents by StrataGraft has not been reported,” the agency warned.
The most common side effects reported with the skin graft include pruritis, blisters, hypertrophic scar, and impaired healing at the treatment site. The agency noted that overall, the product’s safety profile regarding wound related events—including erythema, swelling, local warmth, and wound site infections—”was similar to that of autografting in these studies [used to support the approval.”
StrataGraft is manufactured by Stratatech, a Mallinckrodt company, and was developed in conjunction with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority.
John McKenna, Associate Editor, BreakingMED™
Cat ID: 105
Topic ID: 75,105,730,105,192,725,925