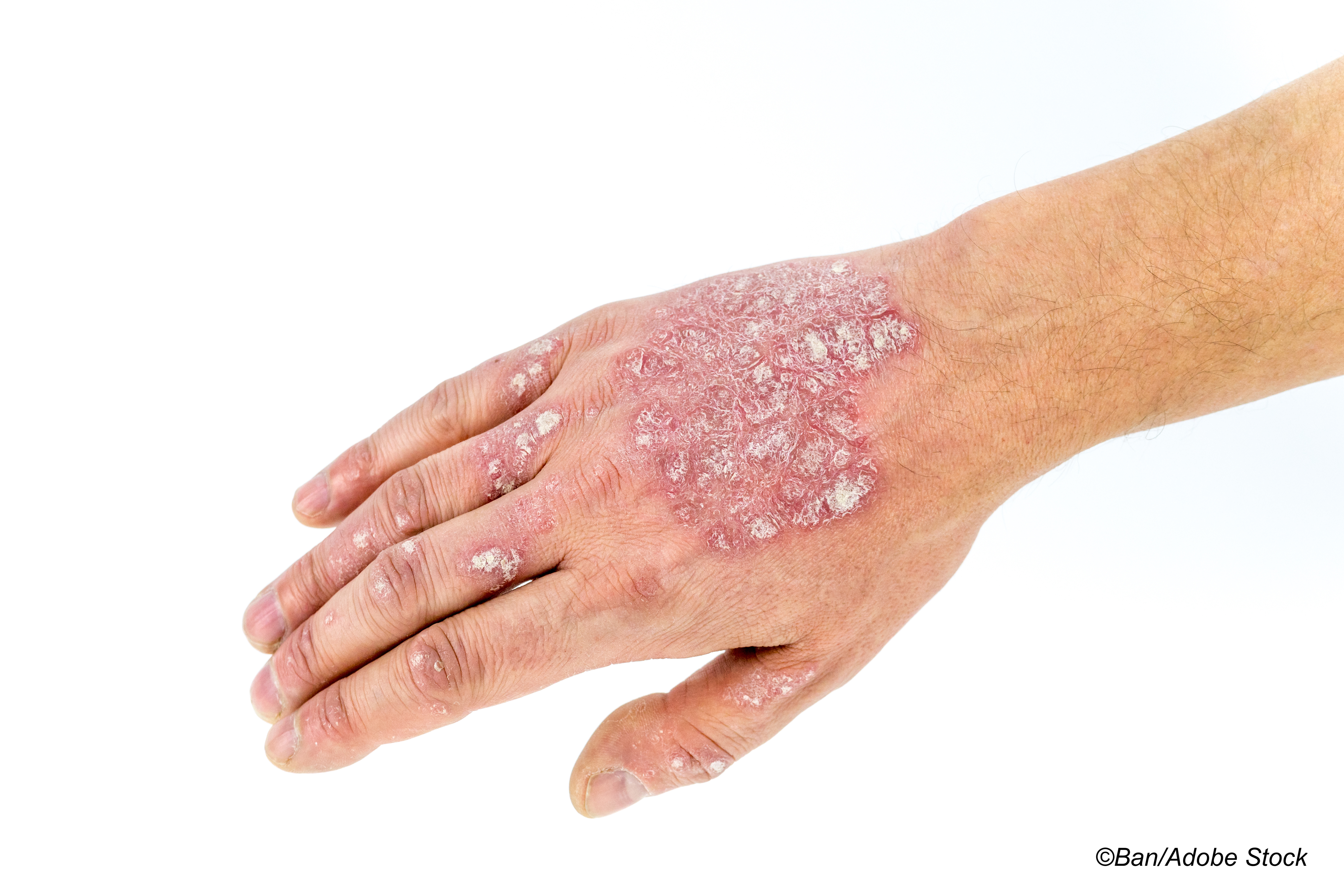
Tapinarof 1% cream reduced the severity of plaque psoriasis over 12 weeks in patients with mild-to-moderate psoriasis but was associated with local adverse events, according to results from two identical phase III randomized studies published in The New England Journal of Medicine.
Still investigational, tapinarof 1% is a first-in-class, once-daily topical therapeutic aryl hydrocarbon receptor (AhR) modulating agent.
“The aryl hydrocarbon receptor is a ligand-dependent transcription factor with roles in the regulation of cytokine and skin-barrier–protein expression and antioxidant activity, which makes it a reasonable therapeutic target for the treatment of inflammatory skin diseases and potentially other immunologic diseases,” explained Mark G. Lebwohl, MD, Dean for Clinical Therapeutics, Icahn School of Medicine at Mount Sinai, and Chairman emeritus, Kimberly and Eric J. Waldman Department of Dermatology, and fellow researchers.
“Tapinarof binds to and activates the aryl hydrocarbon receptor and has been shown in preclinical studies to modulate the expression of type 17 helper T cytokines implicated in psoriasis, including interleukin-17A and interleukin-17F, the skin barrier proteins filaggrin and loricrin, and the aryl hydrocarbon receptor nuclear factor erythroid 2–related factor 2 (Nrf2) transcription factor pathway leading to expression of antioxidant enzyme genes, such as nicotinamide adenine dinucleotide (phosphate) quinone oxidoreductase 1 and heme oxygenase-1, that reduce oxidative stress. Taken together, these findings suggest that the mechanism of action of tapinarof involves immune modulation, skin-barrier normalization, and antioxidant activity,” they added.
In June 2021, tapinarof’s manufacturer, Dermavant, submitted a New Drug Application (NDA) to the FDA seeking approval for tapinarof cream for the treatment of mild, moderate, and severe plaque psoriasis in adult patients.
For the PSOARING 1 and PSOARING 2 trials, Lebwohl and colleagues included 510 and 515 patients with mild-to-severe plaque psoriasis who were randomized to treatment with tapinarof 1% cream or vehicle cream used once daily for 12 weeks. All patients had a baseline Physician’s Global Assessment (PGA) score of 2-4 (mild to severe on a scale of 0-4) and a percent affected total body-surface area of 3%-20%.
Researchers noted that baseline characteristics were similar in both trials, but more patients in the tapinarof group (22.4%) had a duration of disease of less than 5 years.
The primary endpoint of both studies was PGA response in a score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points by week 12. Secondary endpoints included at least a 75% reduction in the Psoriasis Area and Severity Index (PASI) score, a PGA score of 0 or 1 mean change from baseline in percent affected body-surface area, and at least a 90% decrease in PASI scores.
In trial 1, 35.4% of patients treated with tapinarof had a PGA response, compared with 6.0% of those treated with vehicle; in trial 2, these response rates were 40.2% and 6.3%, respectively (P<0.001 for both).
As far as secondary efficacy endpoints, 36.1% of patients treated with tapinarof had a PASI 75 response, compared with 10.2% of those treated with vehicle. In trial 1, this was a difference of 25.9 percentage points (relative rate: 2.8; 95% CI: 1.7-4.5; P<0.001). These PASI 75 response rates were 47.6% and 6.9%, respectively, for a difference of 40.7 percentage points (relative rate: 6.5; 95% CI: 3.7-11.5; P<0.001).
In trial 1, 37.8% of patients treated with tapinarof had a PGA score of 0 or 1 at week 12, compared with 9.9% in the vehicle group (difference: 27.9 percentage points; relative rate: 2.7; 95% CI: 1.6-4.4; P<0.001). In trial 2, these rates were 43.6% and 8.1%, respectively, with a difference of 35.5 percentage points (relative rate: 4.6; 95% CI: 2.7-7.6; P<0.001).
Patients treated with tapinarof had a mean change from baseline to week 12 of -3.5 percentage points in the percentage of total body-surface area affected, compared with –0.2 percentage points in those treated with vehicle (difference: –3.3 percentage points; 95% CI: –4.4 to –2.1; P<0.00). In trial 2, the corresponding change was –4.2 percentage points and 0.1 percentage points, respectively (difference: –4.3 percentage points; 95% CI: –5.2 to –3.5; P<0.001).
PASI 90 responses occurred in 18.8% of the tapinarof group, compared with 1.6% of the vehicle group in trial 1 at week 12 (difference: 17.2 percentage points; relative rate: 8.5; 95% CI: 2.6-284; P<0.001); in trail 2, responses occurred in 20.9% and 2.5% of patients, respectively (difference: 18.4 percentage points; relative rate: 7.2; 95% CI: 2.9-18.4; P<0.001).
In patient-reported outcomes, greater change was seen in total PP-NRS scores from baseline to week 12 in patients treated with tapinarof versus vehicle (–3.6 vs –2.7, respectively) in trial 1, and –3.0 versus –1.4 in trial 2. Sixty percent of patients with baseline PP-NRS scores of at least 4 points had a decrease of at least 4 points by week after treatment with tapinarof, compared with 43.2% of those in the vehicle group in trial 1; in trial 2, the incidence was 56.9% versus 29.6%, respectively.
In trial 1 in the tapinarof group, mean change from baseline to week 12 in Dermatology Life Quality Index total score was –4.6, compared with –2.8 in the vehicle group; in trial 2, corresponding values were –4.4 versus –1.1, respectively. And finally, changes in the Psoriasis Symptom Diary score were –48.5 versus –34.0 and –42.9 versus –18.8.
In all, 50.3% of patients treated with tapinarof experienced adverse events, as did 22.4% of those who received the vehicle cream in trial 1, and in 54.5% versus 26.2%, respectively, in trial 2. No serious treatment-related adverse events occurred in either trial.
Folliculitis occurred in 23.5% of patients treated with tapinarof versus 1.2% of those treated with vehicle in trial 1, and in 17.8% and 0.6% in trial 2. In trial 1, there was one grade 3 adverse event (folliculitis). Contact dermatitis occurred in 5.0% of the tapinarof group versus 0.65% of the vehicle group in trial 1, and in 5.8% and 0%, respectively, in trial 2. One severe occurrence of contact dermatitis was seen in the tapinarof group in trial 2.
Headache occurred in 3.8% of the tapinarof group, compared with 2.4% of the vehicle group in trial 1, and in 3.8% and 0.6%, respectively, in trial 2, with one severe occurrence of headache 30 days after completion of tapinarof in trial 2.
Burning, stinging, and itching was reported by patients as low in both trials, with minimal between-group differences.
According to Lebwohl, there is a need for new nonsteroidal treatments for patients with psoriasis.
“Psoriasis patients with mild to moderate disease are likely to benefit from this treatment,” he told BreakingMED in an email correspondence. “Because tapinarof cream is not a steroid, we do not have to worry about steroid side effects like stretch marks, cataracts, glaucoma, and others.”
“Tapinarof cream is highly effective and efficacy is durable in those who continue on the medication. For those who clear completely, psoriasis remains in remission for approximately four months after discontinuation of the treatment. The treatment is safe even on steroids sensitive sites like the face and intertriginous areas,” Lebwohl concluded.
“Concerns regarding other safety issues have limited the clinical development of drugs that affect AhR for systemic use and have restricted this approach to dermatologic conditions. It has been proposed that AhR agonists can be classified according to their pharmacologic characteristics: selective AhR modulators and rapidly metabolized AhR ligands, the latter being the family to which tapinarof belongs and for which there is not a dioxin-like effect,” wrote Thomas Bieber, MD, PhD, MDRA, of the Department of Dermatology and Allergy, Christine Kühne–Center for Allergy Research and Education, University Hospital of Bonn, Bonn, Germany, in his accompanying editorial.
“In addition to AhR agonists, relatively new compounds such as phosphodiesterase type 4 inhibitors (e.g., roflumilast) and Janus kinase inhibitors (e.g., brepocitinib) are in clinical development and may expand the armamentarium for topical therapy of psoriasis. Third-party payers worldwide and health technology assessment agencies in Europe, with their cost-effectiveness–based decisions for pricing and reimbursement, may present hurdles for broad access to innovative therapies such as tapinarof and other compounds in advanced stages of clinical development for the treatment,” he added.
Study limitations include the lack of endpoint data in about 15%-20% of patients and the use of multiple imputation to adjust, the possible unmasking of active treatment due to local adverse events, and the nongeneralizability to patients outside the United States and Canada (where the two studies were performed) and to patients with other forms of psoriasis.
-
Tapinarof 1% cream once daily was superior to vehicle control in reducing the severity of plaque psoriasis over 12 weeks of treatment, but was also associated with local adverse events and headache.
-
Researchers of this investigational treatment call for more studies to assess the safety and efficacy of tapinarof and compare it with existing treatment.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
This study was supported by Dermavant Sciences.
Lebwohl has received grants/contracts from AbbVie, Amgen, Arcutis, Avotres Therapeutics, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly and Company, Incyte Corp., Janssen Research & Development LLC, Ortho Dematologics, Regeneron Pharmaceuticals, and UCB. He has served as a consultant for Aditum Bio, Almirall, Altrubio, Anaptysbio, Arcutis, Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, Biomx, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, DR. Reddy’s Laboratories, Evelo Biosciences, Evoimmune Inc., Facilitation of Internatial Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima LTD., LEO Pharma AS, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica.
Bieber reported personal fees from AbbVie, Almirall, Arena, Asana, ASLAN, Bayer Health, Boehringer-Ingelheim, Domain Therapeutics, Galderma, Incyte, Jansen, Kymab, LEO Pharma LG Chemical, Lilly,MenloTx,OM Pharma,Pfizer,Pierre-Fabre, RAPT, Sanofi-Genzyme, UCB, Regeneron, and from Dermavant, outside the submitted work.
Cat ID: 10
Topic ID: 75,10,730,10,105,192,919,925