Treatment with omecamtiv mecarbil—a direct activator of cardiac myosin—effected significant improvements in outcomes among patients with severe heart failure (HF), which included a 20% reduction in relative risk for time to first HF event or cardiovascular (CV) death. Researchers of this post-hoc analysis of data from the GALACTIC-HF study concluded that omecamtiv mecarbil may be a potential treatment for patients with severe HF in whom current treatment options are limited.
Their results were published in JAMA Cardiology.
“As HF progresses, many patients become progressively intolerant of neurohormonal blockade with β-blockers or renin-angiotensin-aldosterone system modulators because of hypotension or kidney dysfunction, limiting their options for medical therapy. Selected patients with severe HF may be candidates for other therapies, such as cardiac transplant or mechanical cardiac support, but these therapies are costly and highly invasive and have limited availability. Intravenous inotropic therapy can be used for palliation of symptoms among selected patients but may be associated with increased mortality. Thus, there is a clear unmet need for effective and safe long-term medical therapies for the treatment of patients with more severe stages of HF,” wrote G. Michael Felker, MD, MHS, of Duke Clinical Research Institute, Durham, North Carolina, and fellow researchers.
Felker and colleagues conducted this post-hoc analysis to assess the efficacy and safety of omecamtiv mecarbil in patients with severe HF who were enrolled in the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF).
“Omecamtiv mecarbil is a direct activator of cardiac myosin that increases systolic ejection time and stroke volume, improves ventricular remodeling, and decreases natriuretic peptide concentrations in patients with [heart failure with reduced ejection fraction] HFrEF,” they explained.
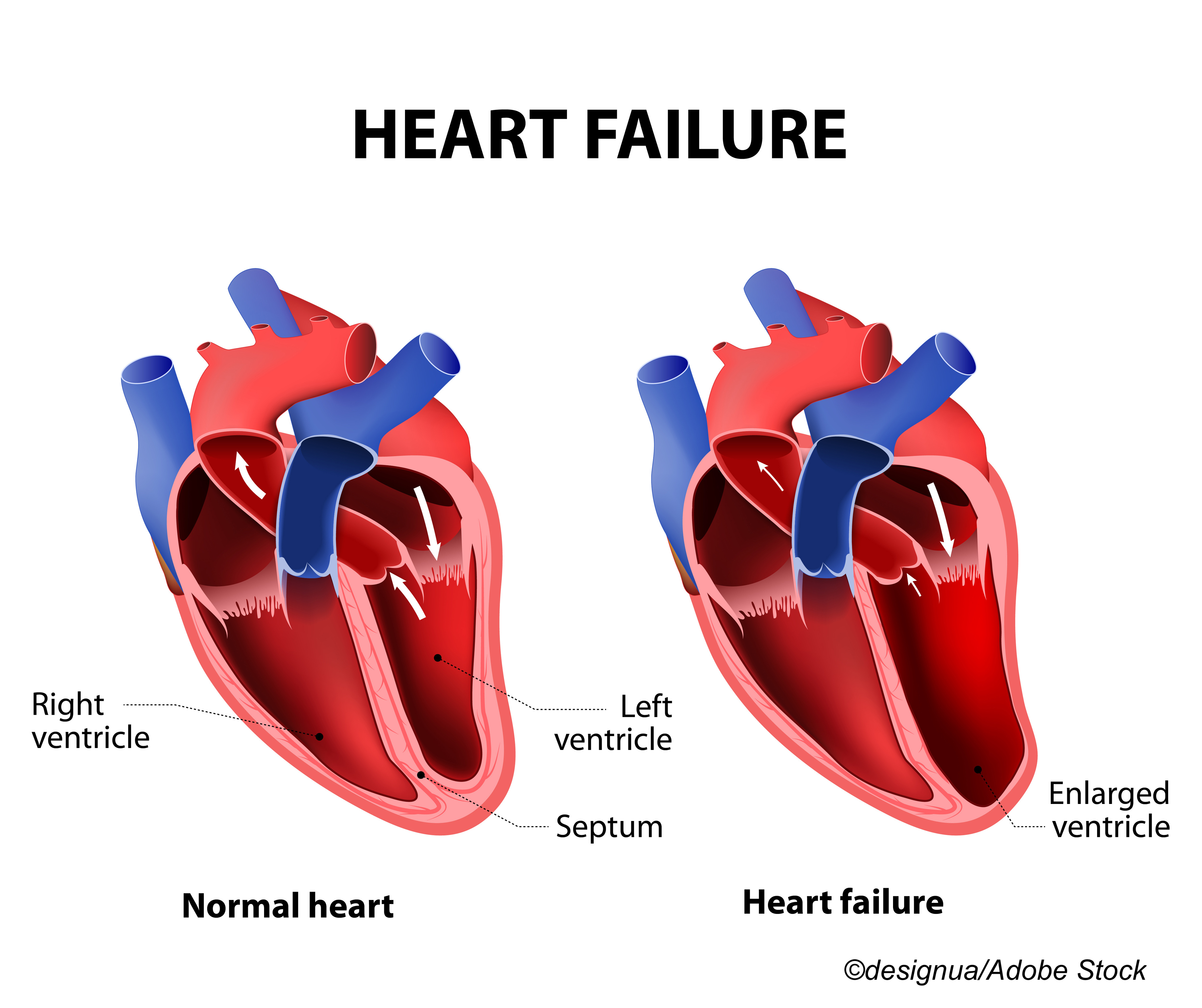
In all, 8,232 patients with symptomatic HF and a left ventricular ejection fraction (LVEF) of ≤35% were randomized to treatment with omecamtiv mecarbil or placebo and were followed for a median of 21.8 months. Severe HF was defined by researchers as the presence of all of the following: New York Heart Association symptom class III to IV, LVEF of ≤30%, and HF hospitalization within the past 6 months. In all, 1,106 patients with severe HF were treated with omecamtiv mecarbil, as were 3,014 without severe HF, and compared with 1,152 severe HF patients and 2,960 without severe HF treated with placebo.
The primary endpoint was time to first HF event or cardiovascular (CV) death, and patients with severe HF treated with omecamtiv mecarbil demonstrated a significant 20% risk reduction for this endpoint (HR: 0.80; 95% CI: 0.71-0.90), compared with those without HF treated with omecamtiv mecarbil, who experienced no significant risk reduction (HR: 0.99; 95% CI: 0.91-1.08; P=0.005 for interaction). Similar results were seen for the secondary endpoint of CV death in patients with severe HF treated with omecamtiv mecarbil (HR: 0.88; 95% CI: 0.75-1.03) compared with patients without severe HF (HR: 1.10; 95% CI: 0.97-1.25; P=0.03 for interaction).
When researchers assessed event rates and treatment effects of omecamtiv mecarbil according to which and how many severe HF criteria were met, the greatest benefits of treatment with omecamtiv mecarbil occurred in patients meeting all three severe HF criteria, the group with the highest overall risk.
“The combination of a 20% relative risk reduction in the primary end point in the context of high baseline risk translated to an absolute risk reduction of 8.3 events per 100 patient-years (number needed to treat=12; 34.3 events per 100 patient-years in the omecamtiv mecarbil group versus 42.6 events per 100 patient-years in the placebo group; P<0.001). These results were broadly consistent across a variety of other secondary outcomes from the GALACTIC-HF clinical trial,” wrote Felker et al.
Patients with severe HF treated with omecamtiv mecarbil had no significant changes in blood pressures, kidney function, or potassium levels compared with placebo, but they were more likely to have treatment-emergent serious adverse events (TESAEs) compared with patients without severe HF (68.1% versus 55.0%, respectively). These TESAEs, however, were similar when researchers compared patients with severe HF who received omecamtiv mecarbil and those receiving placebo (67.3% versus 68.8%; P=0.43).
Patients with severe HF treated with omecamtiv mecarbil were more likely, however, to experience myocardial infarction compared with those who received placebo (3.8% versus 2.5%, respectively; P=0.08), but had fewer index strokes (1.6% versus 2.7%; P=0.08).
“In this issue of JAMA Cardiology, Felker et al not only provide an important statement about the potential benefits and risks of omecamtiv mecarbil therapy but, more importantly, provide a clinically evident demarcation of a potentially new stage of HF, specifically patients with HFrEF who are receiving effective guideline-directed medical therapy (i.e., patients with stage C HF) but have progressive or severe symptoms that do not yet reach the threshold for advanced therapies (i.e., stage D HF). This demarcation is not a quirk of recruitment within a large clinical trial but a true, common, and actionable clinical dilemma,” wrote Clyde W. Yancy, MD, MSc, of Northwestern University Feinberg School of Medicine, Chicago, and colleagues in an accompanying editorial.
“Extending the interpretation of the GALACTIC-HF data, the current analysis also makes evident that there is a substantial proportion of patients with stage C HFrEF for whom the addition of omecamtiv mecarbil is not likely to be of tangible benefit. Given the amount of evidence-based therapies for HFrEF, better calibration of use, particularly through identification of patients for whom the benefits are minimal and the cost or burden can be avoided, represents an excellent step forward and aids our efforts to achieve optimal therapy for patients with HFrEF,” they concluded.
Study limitations include its post hoc design and the exclusion of patients with estimated glomerular filtration rates ˂20 mL/min/1.73 m2 and those receiving dialysis at the time of screening.
-
In patients with severe heart failure (HF), omecamtiv mecarbil therapy effected a clinically meaningful reduction in the composite end point of time to first HF event or cardiovascular death.
-
These results suggest that omecamtiv mecarbil may be a potential treatment for patients with severe HF in whom current treatment options are limited.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
The Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) clinical trial was funded by Amgen, Cytokinetics, and Servier Laboratories.
Felker reported receiving grants and personal fees from Amgen and Cytokinetics during the conduct of the study; grants from Bayer and Merck & Co; and personal fees from American Regent, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Medtronic, and Novartis outside the submitted work.
Yancy reported having a spouse who is employed by Abbott Laboratories outside the submitted work.
Cat ID: 3
Topic ID: 74,3,730,3,192,925