Treatment with a chimeric antigen receptor (CAR) T-cell therapy, idecabtagene vicleucel, led to frequent and deep responses in patients with mostly triple drug-class resistant, relapsed and refractory multiple myeloma, the phase II KarMMa study found.
At a median follow-up of 13.3 months (range 0.2-21.2 months), 73% (95% CI, 66-81%; P<0.001) of 128 heavily pretreated patients achieved an overall response — defined as a partial response or better — to treatment with a single infusion of one of three target doses of idecabtagene vicleucel, Nikhil Munshi, MD, Dana-Farber Cancer Institute, Boston, and co-investigators reported in The New England Journal of Medicine.
One-third of patients achieved a complete response or better — defined as a complete and a stringent complete response — and 52% achieved a very good partial response or better. Moreover, minimal residual disease (MRD)-negative status was confirmed in 26% of all patients who received treatment, as well as in 79% of the smaller group of patients who achieved a complete response or better to idecabtagene vicleucel infusion.
The fact that approximately one-quarter of patients achieved MRD-negative responses highlights the depth of responses induced by idecabtagene vicleucel infusion, as investigators observed.
In September 2020, the FDA granted prority review for a biologic license application for idecabtagene vicleucel.
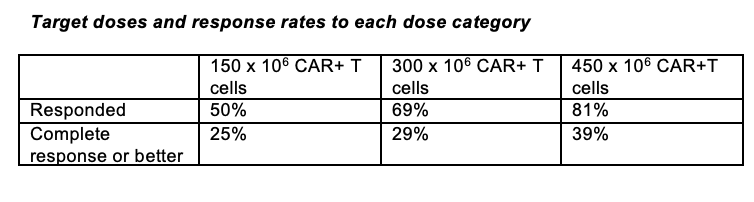
“No standard of care has been established for patients who have disease progression despite receiving the three main classes of myeloma therapy… and outcomes are poor,” the study authors wrote. “…Results of the KarMMa study support substantial antitumor activity for idecabtagene vicleucel across a target dose range of 150×106 to 450×106 CAR+ T cells. The 450 x 106 dose appeared to be somewhat more effective than the other doses.”
The median age of the treatment group was 61 years (range, 33-78 years) and the median time since diagnosis was 6 years (range, 1-18 years).
Slightly over half had a high tumor burden, 39% had extramedullary disease and 35% had a high-risk cytogenetic abnormality.
Patients had received a median of six previous treatments (range, 3-16) and 94% had undergone a previous autologous hematopoietic stem-cell transplant.
Almost all patients (84%) had triple drug-refractory disease to the three main treatments for multiple myeloma, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody.
Lymphodepletion with fludarabine, 30 mg/m2 per day plus cyclophosphamide, 300 mg/m2 per day, was administered on three consecutive days followed by two days of rest prior to idecabtagene vicleucel infusion. “Patients received target doses of 150×106, 300×106, or 450×106 CAR-positive (CAR+) T cells.”
Responses improved as the dose of the CAR+ T cell therapy increased in patients who achieved any response to treatment as well as those who achieved a complete response or better, although to a lesser extent.
Among patients who achieved either a complete or a stringent complete response, 79% also achieved MRD-negative status, and median time to first response was 1.0 months (range, 0.5-8.8 months) while the median time to a complete response or better was 2.8 months (range 1.0-11.8 months).
The median duration of response was 10.7 months (95% CI, 9.0-11.3 months) overall but it was slightly longer at a median of 11.3 months (95% CI, 10.3-11.4 months) among patients who received the highest dose of idecabtagene vicleucel.
“[R]esponse duration increased with depth of response,” investigators noted, reaching a median duration of 19.0 months (95% CI, 11.3 months to could not be estimated) in patients achieving a complete or stringent complete response.
Median progression-free survival (PFS) was 8.8 months overall (95% CI, 5.6-11.6 months) but it was again somewhat longer in patients who received the highest dose of idecabtagene vicleucel at 12.1 months (95% CI, 8.8-12.3 months). Median overall survival (OS) was 19.4 months (95% CI, 18.2 months to could not be estimated), with some 78% of all treated patients still being alive at 12 months.
Idecabtagene vicleucel also durably persisted in blood, with over one-third of evaluable patients having detectable CAR+ T cells at 12 months of follow-up, although the presence of CAR+ T cells did not protect patients from recurrence.
“Adverse events were reported in all 128 patients treated with ide-cel, with grade 3 or 4 eventsoccurring in 127 patients (99%). Most adverse events, with the exception of hypogammaglobulinemia and infections, occurred within the first 8 weeks after infusion.”
The cytokine release syndrome also occurred in 84% of patients although it was largely confined to grades 1 or 2, with only 6% of patients developing higher grades of the syndrome.
“A total of 44 treated patients (34%) died during the study, with most deaths (27) attributed by the investigator to complications of myeloma progression (Table S12). Three patients (2%) died within 8 weeks after ide-cel infusion from ide-cel–related adverse events (bronchopulmonary aspergillosis, gastrointestinal hemorrhage, and cytokine release syndrome). One patient (1%) died between 8 weeks and 6 months from an ide-cel–related adverse event (cytomegaloviral pneumonia). Five patients (4%) died after 6 months.”
-
Treatment with an investigational CAR+ T cell therapy induced frequent and deep responses in mostly triple drug-class resistant relapsed and refractory multiple myeloma with high risk features.
-
All patients reported adverse events, with the majority of them being hematologic in nature, although infections were also common.
Pam Harrison, Contributing Writer, BreakingMED™
The trial was supported by bluebird bio and Celgene, a Bristol-Myers Squibb company.
Munshi disclosred consultant agreements with Adaptive Biotechnology, Amgen, Bristol-Myers Squibb, Janssen Biotech, Karyopharma Therapeutics, legend, Oncopep, and Takeda, as well as serving on a data safety monitoring board for AbbVie Biotherpeutics and stock ownership in Oncopep.
Cat ID: 118
Topic ID: 78,118,730,118,468,192,925


