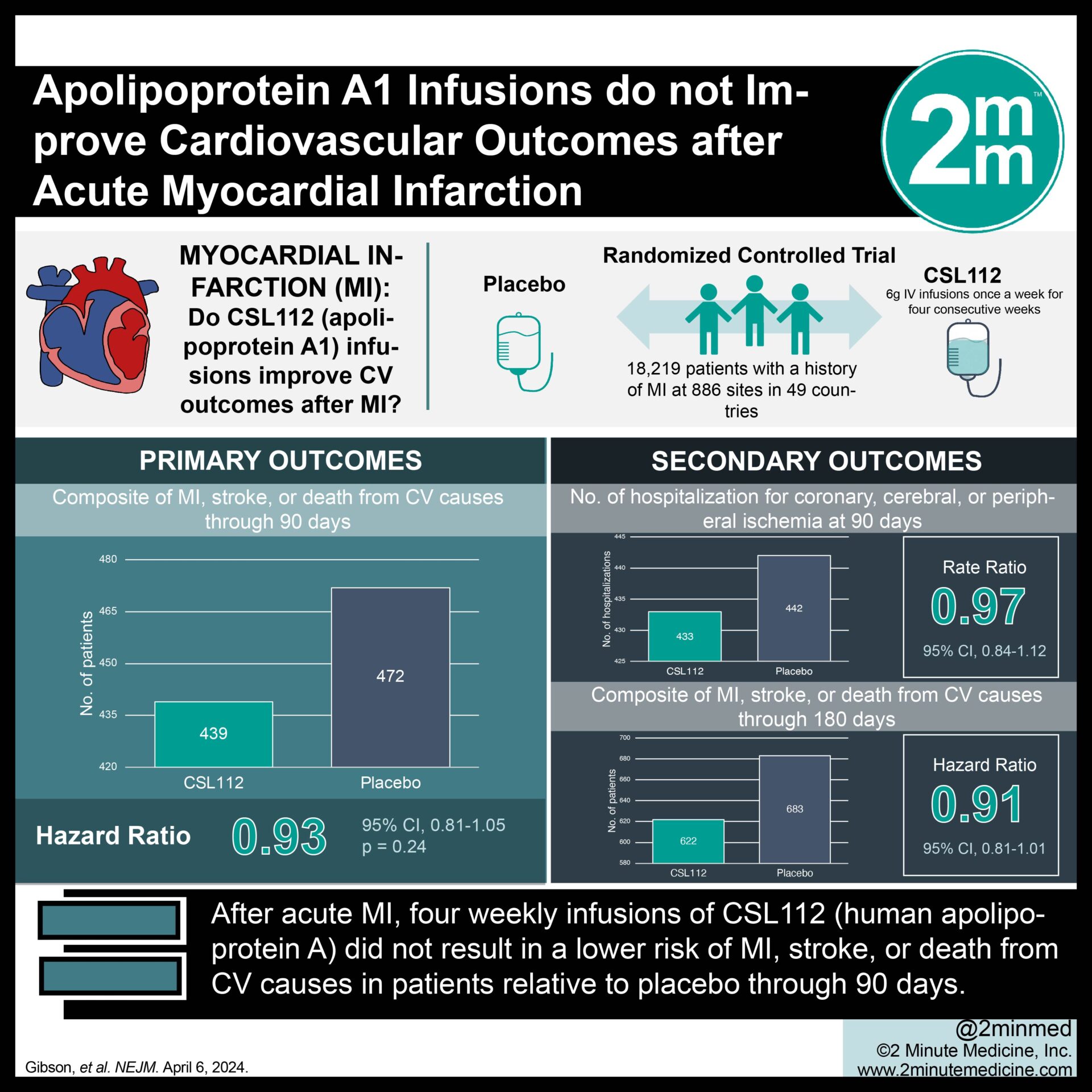
Background In patients with acute ischemic stroke, little is known regarding the frequency of abnormal ECG findings other than atrial fibrillation and their association with cardiovascular outcomes. We aim to analyze the frequency and type of abnormal ECG findings, subsequent changes in medical treatment, and their association with cardiovascular outcomes in patients with acute ischemic stroke. Methods and Results In the investigator-initiated multicenter MonDAFIS (impact of standardized monitoring for detection of atrial fibrillation in ischemic stroke) study, 3465 patients with acute ischemic stroke or transient ischemic attack and without known atrial fibrillation were randomized 1:1 to receive Holter-ECG for up to 7 days in-hospital with systematic evaluation in a core cardiology laboratory (intervention group) or standard diagnostic care (control group). Outcomes included predefined abnormal ECG findings (eg, pauses, atrial fibrillation, brady-/tachycardias), medical management in the intervention group, and combined vascular end point (recurrent stroke, myocardial infarction, major bleeds, or all-cause death) and mortality at 24 months in both randomization groups. Predefined abnormal ECG findings were detected in 326 of 1693 (19.3%) patients in the intervention group. Twenty of these 326 patients (6.1%) received a pacemaker, and 62 of 326 (19.0%) patients had newly initiated or discontinued β-blocker medication. Discontinuation of β-blockers was associated with a higher death rate in the control group than in the intervention group during 24 months after enrollment (adjusted hazard ratio, 11.0 [95% CI, 2.4-50.4]; =0.025 for interaction). Conclusions Systematic in-hospital Holter ECG reveals abnormal findings in 1 of 5 patients with acute stroke, and mortality was lower at 24 months in patients with systematic ECG recording in the hospital. Further studies are needed to determine the potential impact of medical management of abnormal ECG findings. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT02204267.