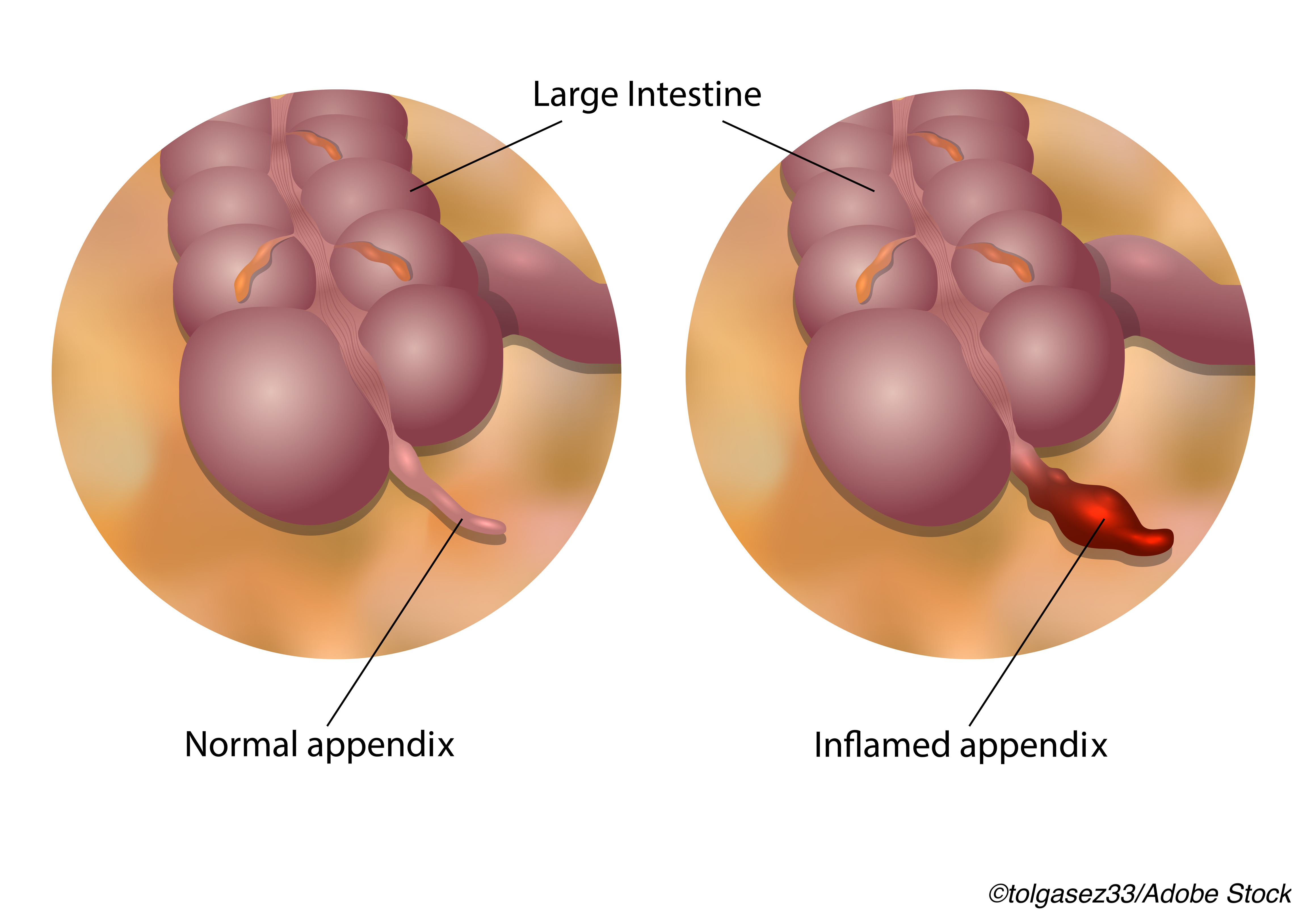
Antibiotic monotherapy for 7 days straight led to treatment success rates of over 65% in adults with uncomplicated acute appendicitis; however, this regimen failed to achieve non-inferiority versus a course of IV antibiotics followed by oral drugs, according to results of the APPAC II trial.
In over 500 patients with 1-year of follow-up data, the treatment success rate was 70.2% (1-sided 95% CI 65.8% to infinity) for patients treated with 7 days of oral moxifloxacin versus 73.8% (1-sided 95% CI 69.5% to infinity) for patients treated with 2 days of IV ertapenem followed by 5 days of levofloxacin and metronidazole, reported Paulina Salminen, MD, PhD, of Turku University Hospital in Turku, Finland, and co-authors.
The difference was −3.6% (1-sided 95% CI −9.7% to infinity) for a P-value of 0.26 for non-inferiority. The confidence limit exceeded the non-inferiority margin, the authors wrote in JAMA.
While noninferiority wasn’t met, “the majority of patients avoided appendectomy in both groups… (70.2%) with uncomplicated acute appendicitis were successfully treated with oral moxifloxacin monotherapy and did not experience major complications attributable to a trial of oral-only antibiotics,” Salminen and co-authors pointed out.
Of course, the findings need to be viewed in relation to previous research, such as initial and follow-up results (5 year and 7 year) from the 2015 APPAC trial, data from the 2020 Midwest Pediatric Surgery Consortium (MWPSC) study, and the 2020 CODA Collaborative trial, argued Peter C. Minneci, MD, MHSc, and Katherine J. Deans, MD, MHSc, both of Nationwide Children’s Hospital in Columbus, Ohio, in an editorial accompanying the study. Minecci and Deans are MWPSC investigators.
“The results from these large trials are remarkably consistent, with a success rate for antibiotic treatment of appendicitis of approximately 70%, less disability, and similar treatment-associated complications,” they pointed out. “Nonoperative management and surgery for the treatment of uncomplicated appendicitis should both be offered as part of an informed decision-making process in clinical care.”
While the editorialists noted that the trial was pre-pandemic, taking a noninvasive or less invasive treatment approach now is particularly important “because it would allow for patients to be treated without admitting them to the hospital. This would help relieve strain on the health care system by limiting inpatient bed use during a time of shortage and alleviate patient anxiety related to Covid-19 exposure during a hospital admission,” Minneci and Deans said.
However, they cautioned that this care path may be easier said than done due to possible barriers, including “persistent dogma related to the inevitability of acute appendicitis progressing in severity if untreated with an operation; physicians, trainees, and other medical staff being unfamiliar with the available evidence to support both treatment choices; patients and families being unfamiliar with nonoperative management as a potential alternative to appendectomy; and the challenge of integrating a shared decision-making process into the acute care setting.”
They suggested using the decision aid and standardized script language laid out in the MWPSC report — and discussed in an accompanying invited commentary — to manage some of these issues. Other researchers have also offered ways to manage appendicitis diagnosis and management.
The APPAC trial was carried out in nine Finnish hospitals from April 2017-Nov. 2018 in 599 patients (mean age 36; 44% women) with CT-confirmed uncomplicated acute appendicitis. Follow-up was done through Nov. 2019. Patients with complicated acute appendicitis based on CT were excluded.
However, Salminen’s group noted that [four] patients… were incorrectly enrolled in the study despite meeting exclusion criteria on primary CT findings, and 136 eligible patients who should have been evaluated for enrollment were not,” which was a study limitation.
Enrolled patients were randomized to receive oral moxifloxacin (400 mg/d) for 7 days or IV ertapenem (1 g/d) for 2 days followed by oral levofloxacin (500 mg/d) and metronidazole (500 mg three times daily) for 5 days.
The trial’s primary endpoint was treatment success (≥65%) for both groups and was defined as “discharge from hospital without surgery and no recurrent appendicitis during 1-year follow-up, and to determine whether oral antibiotics alone were non-inferior to intravenous and oral antibiotics, with a margin of 6% for difference,” the authors explained.
They reported that 9.2% of those in the monotherapy group underwent an appendectomy during the primary hospitalization and 20.7% underwent an appendectomy during 1-year follow-up. In the other group, 7.6% underwent appendectomy during primary hospitalization and 18.5% underwent appendectomy during the 1-year follow-up for suspected appendicitis recurrence. Among all of these patients (n=49), 18 in the monotherapy group and 11 in the other group were found to have complicated appendicitis at the time of the procedure, leading to a “true primary failure rates” of 6.1% and 3.8%, respectively, for a difference of 2.3% (90% CI −0.7% to 5.2%, P=0.25).
Salminen and co-authors also found that there was no statistically significant difference between treatment groups in terms of length of hospital stay, or sick leave, or visual analog scale (VAS 1-10) scores for pain at discharge (1.0 for both groups), 1 week (0 for both), and 2 months (0 for both). Also, among the patients who had surgery, 2.6% were diagnosed with an appendiceal tumor. Finally, there was no mortality during the 1-year follow-up, they said.
Other study limitations included the fact that moxifloxacin is a broad-spectrum antibiotic that carries a risk for antibiotic resistance. Also, “the predefined difference of 0% and the noninferiority margin for clinical importance of 6% in the sample size calculations between the study groups were set somewhat arbitrarily because no previous trials were available comparing oral and intravenous antibiotics for uncomplicated acute appendicitis,” the authors explained.
But as with Minneci and Deans, Salminen and co-authors highlighted that “Avoiding hospitalization for the 70.2% of patients with acute appendicitis who were successfully treated with oral antibiotics might reduce the risk of disease exposure to Covid-19 for these patients and free hospital resources at a time of bed capacity shortages. These current conditions may warrant what is considered treatment success for oral antibiotic treatment of acute appendicitis.”
-
Patients with acute uncomplicated appendicitis treated with oral antibiotics alone met the prespecified threshold for treatment success versus a two-drug regimen — 7 days of oral moxifloxacin or 2 days IV ertapenem followed by 5 days of levofloxacin and metronidazole — but failed to demonstrate non-inferiority relative to systemic antibiotics followed by oral antibiotics.
-
In the APPAC II trial, 70.2% of the patients with uncomplicated acute appendicitis avoided appendectomy, were successfully treated with oral moxifloxacin monotherapy, and did not experience major complications.
Shalmali Pal, Contributing Writer, BreakingMED™
Salminen reported support from the Mary and Georg C. Ehrnrooth Foundation, the Sigrid Juselius Foundation, the Finnish Academy, Turku University Hospital, and the EVO Foundation, as well as relationships with Merck and Orion Pharma. Co-authors reported support from, and/or relationships with, the Orion Research Foundation, the Gastroenterological Research Foundation, Turku University Hospital, the Mary and Georg C. Ehrnrooth Foundation, the Finnish Medical Foundation, the European Society of Clinical Microbiology and Infectious Diseases, Pfizer, Merck Sharp & Dohme, and Roche.
Minneci and Deans reported no relationships relevant to the contents of this paper to disclose.
Cat ID: 188
Topic ID: 77,188,730,188,255,925