Two studies published in Lancet Gastroenterology and Hepatology illustrate potential advances in treating non-alcoholic fatty liver disease (NAFLD).
In the first study, researchers led by Stephen A. Harrison, MD, Summit Clinical Research, San Antonio, Texas, showed that the blood-based diagnostic NIS4 was effective in non-invasively diagnosing non-alcoholic steatohepatitis (NASH) and liver fibrosis in patients with suspected NAFLD.
In the second study, Takaomi Kessoku, MD, of the Department of Gastroenterology and Hepatology at Yokohama City University Graduate School of Medicine in Yokohama, Japan, and colleagues found that the use of the laxative drug lubiprostone improved intestinal permeability and reduced the level of liver enzymes in patients with NAFLD with constipation.
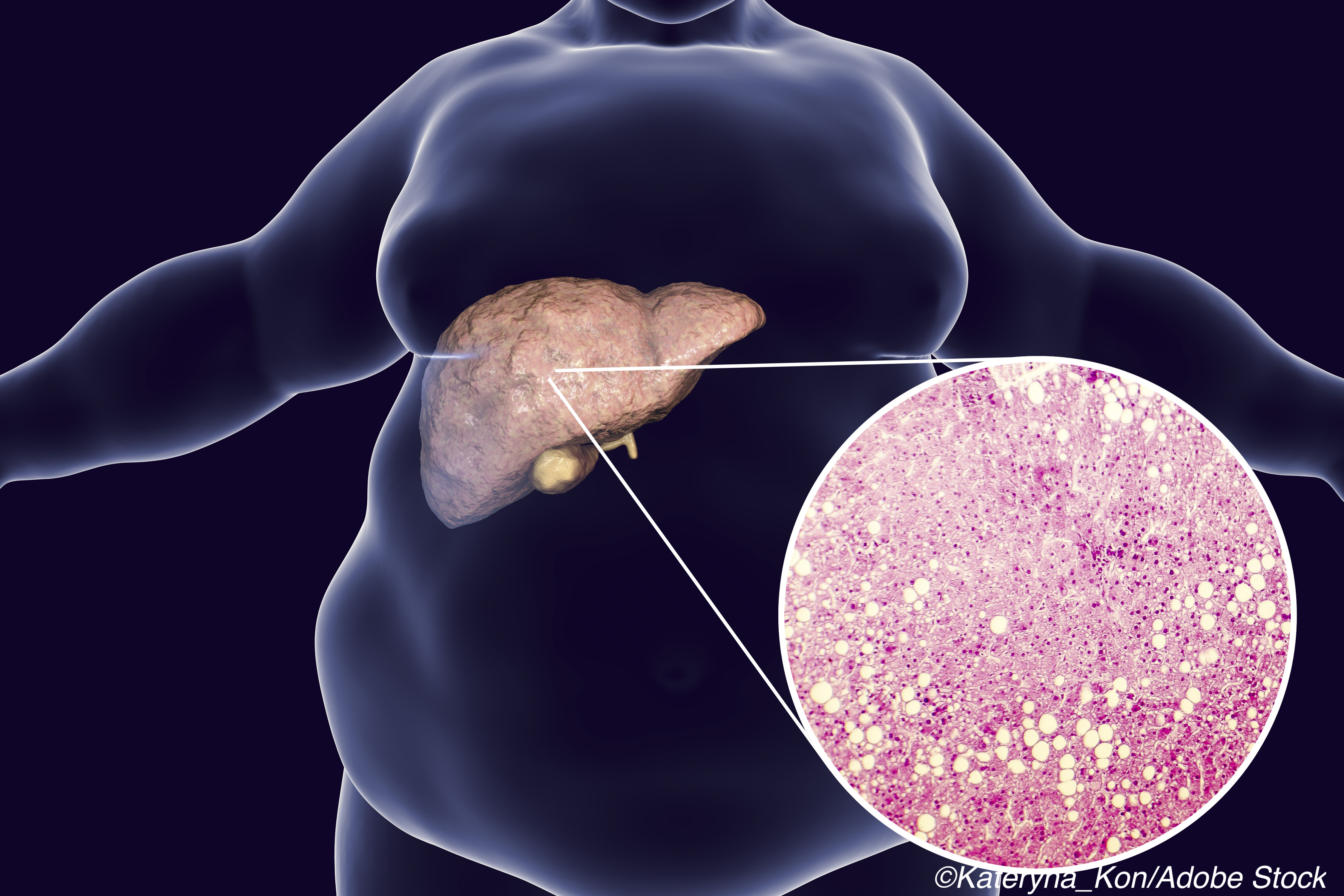
As explained by the authors of each of these studies, NAFLD is highly prevalent world-wide, impacting up to 25% of the general adult population. NASH is a severe form of NAFLD, and if not diagnosed and treated early on it can progress to cirrhosis, liver failure, or hepatocellular carcinoma — in fact, NASH is the leading cause of liver transplantation in the United States.
It is therefore critical to identify patients at higher risk for these clinical scenarios. Currently, liver biopsy is the standard way of diagnosing NASH in patients with clinical risk factors for the disease. However, liver biopsy is invasive, costly, and entails risks. “Providing a non-invasive alternative to liver biopsy will therefore be essential to facilitate improved patient diagnosis, management, and future treatment access in routine clinical practice, and might eventually reduce the morbidity and mortality associated with this disease,” wrote Harrison and colleagues.
The blood-based diagnostic test NIS4 quantitatively measures four independent biomarkers —miR-34a-5p, alpha-2 macroglobulin, YKL-40, and glycated hemoglobin — to produce a score that identifies patients with at-risk NASH (NAFLD disease activity score ≥4 and fibrosis stage ≥2).
Harrison and colleagues validated NIS4 against the liver biopsy reference standard in two independent patient cohorts (702 individuals) selected according to criteria similar to the future intended use population. The two cohorts were combined into a pooled validation cohort.
The authors found that NIS4 “consistently showed high diagnostic performance and low misclassification rates to rule in and rule out at-risk NASH compared with other diagnostic approaches.” Specifically, patients with a NIS4 value less than 0.36 were classified as not having at-risk NASH with 81.5% (95% CI 76.9–85.3) sensitivity, 63.0% (57.8–68.0) specificity, and a negative predictive value of 77.9% (72.5–82.4), while patients with a NIS4 value of more than 0.63 were classified as having at-risk NASH with 87.1% (83.1–90.3) specificity, 50.7% (45.3–56.1) sensitivity, and a positive predictive value of 79.2% (73.1–84.2).
“This non-invasive test is expected to increase the rate of diagnosis of patients with potentially deleterious outcomes, and thereby benefit those in need of specific management, including regular monitoring and pharmacotherapy,” Harrison and colleagues observed.
In the study evaluating lubiprostone, Kessoku and colleagues noted that evidence suggests there is a close linkage between the intestine and liver diseases such as NAFLD, and disease progression is associated with increased intestinal permeability.
Lubiprostone is normally used to treat chronic constipation and irritable bowel syndrome with constipation. Kessoku and colleagues reported that the drug has been shown to improve intestinal permeability in healthy volunteers and hypothesized it could be a therapeutic option for treating NAFLD.
This randomized, double-blind, placebo-controlled, phase IIa trial included patients (aged 20–85 years) with NAFLD and constipation, alanine aminotransferase (ALT) at least 40 U/L, liver stiffness (≤6.7 kPa), and hepatic fat fraction at least 5.2%. Of the 150 patients included for analysis, 55 were assigned to receive 24 μg lubiprostone, 50 to receive 12 μg lubiprostone, and 45 to receive placebo. The primary endpoint of the trial was the absolute change in ALT level from baseline after 12-week lubiprostone treatment compared with placebo.
The authors observed a greater decrease in the absolute ALT levels from baseline to 12 weeks in both the 24 μg and 12 ug lubiprostone groups than in the placebo group. They also found lubiprostone improved intestinal permeability, endotoxemia, and liver fat content.
As for adverse events, about one-third of patients in the 24 ug group had at least one, compared to 6% of patients in the 12 ug group, and 7% in the placebo group. The most commonly reported adverse event across all groups was diarrhea (14%), followed by nausea (4%), and vomiting (2%).
“Manipulation of intestinal permeability induced by lubiprostone might be a potential treatment approach for NAFLD,” Kessoku and colleagues concluded. “Further studies are necessary to better define both the efficacy and tolerability of lubiprostone in patients with NAFLD without constipation.”
-
The blood-based diagnostic NIS4 was effective in diagnosing non-alcoholic steatohepatitis (NASH) and liver fibrosis in patients with suspected NAFLD and represents a non-invasive alternative to liver biopsy.
-
The laxative drug lubiprostone may be a potential treatment approach for NAFLD, according to results from a phase IIa trial.
Michael Bassett, Contributing Writer, BreakingMED™
Harrison reports grants from Bristol-Myers Squibb, Pfizer, Second Genome, Tobira/Allergan, and Genentech; grants, personal fees, and stock or equity from Akero, Axcella Health, Cirius Therapeutics, Galectin Therapeutics, Genfit, Madrigal Pharmaceuticals, Metacrine, NGM Biopharmaceuticals, and NorthSea Therapeutics; grants and personal fees from CiVi Biopharma, CymaBay Therapeutics, Conatus Pharmaceuticals, Galmed Pharmaceuticals, Gilead Sciences, Hepion Pharmaceuticals, HighTide Therapeutics, Intercept Pharmaceuticals, Novartis, Novo Nordisk, Sagimet Biosciences, and Viking Therapeutics; personal fees and stock or equity from HistoIndex; and personal fees from Altimmune, Blade Therapeutics, Chronic Liver Disease Foundation, Corcept Therapeutics, Echosens, Foresite Labs, Gelesis, Indalo Therapeutics, Innovate Pharma, IQVIA, Lipocine, Medpace, Perspectum, Poxel, Prometheus, ProMetic Life Sciences, Terns Pharmaceuticals, and Ridgeline Therapeutics outside the submitted work.
Cat ID: 111
Topic ID: 77,111,111,19,192,925