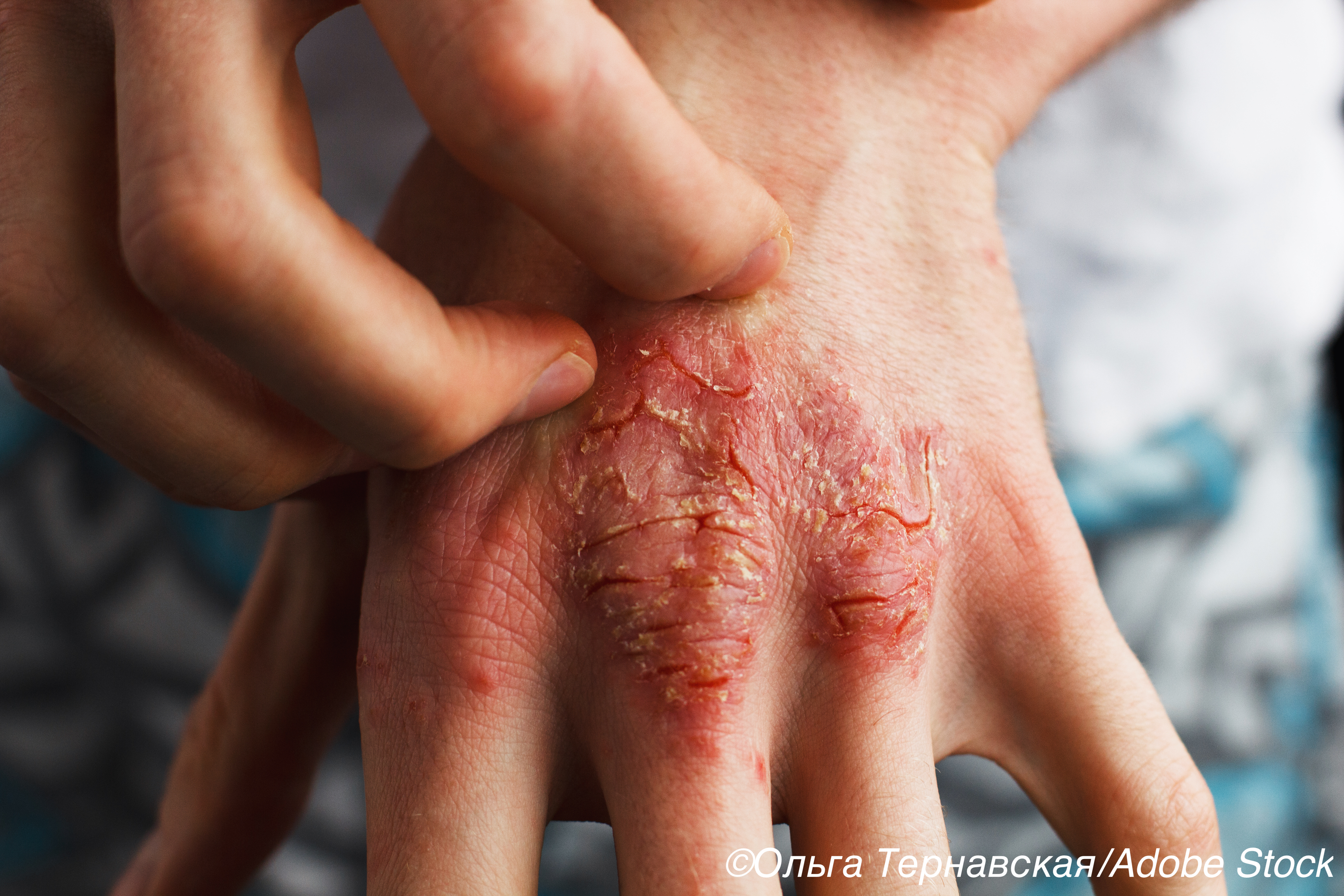
A topical version of a phosphodiesterase type 4 (PDE-4) inhibitor was significantly more effective than placebo for reducing psoriasis severity in a 12-week, phase II clinical trial.
At 6 weeks, more patients with chronic plaque psoriasis treated with once-daily roflumilast cream at 0.3% or 0.15% strength achieved clear or almost clear skin: 28% (0.3%) and 23% (0.15%). Just 8% of patients in the placebo vehicle cream arm of the trial reached this primary outcome.
The study findings were published online July 15 in the New England Journal of Medicine.
At week 8, patients treated with roflumilast 0.3% had achieved improvements in skin clearance similar to those reported in clinical trials for topical glucocorticoids, wrote researcher Mark G. Lebwohl, MD, of Icahn School of Medicine at Mount Sinai, New York City, and colleagues.
The researchers cautioned, however, that no conclusions about the relative efficacy of roflumilast cream versus topical glucocorticoids could be drawn from their historical comparison due to differences in trial design.
They noted that high-potency topical glucocorticoids are a main treatment option for chronic plaque psoriasis, but their use is generally limited to no more than 2 to 8 weeks due to the potential for long-lasting adverse events.
Topical vitamin D derivatives are also used to treat psoriasis, but they are less effective than topical glucocorticoids. Topical calcineurin inhibitors have also been used off-label to treat facial and intertriginous psoriasis, but their use has been associated with an increased risk for cancer and they now carry a black box label warning of this risk.
“Phosphodiesterase type 4 is an enzyme that maintains intracellular levels of cyclic adenosine monophosphate (cAMP) and mediates biologic responses to extracellular stimuli in numerous cell types, including immune cells,” Lebwohl and colleagues wrote.
Studies have shown PDE-4 activity to be greater in psoriatic skin than in healthy skin. Inhibition of PDE-4 results in down-regulation of immune modulators, including TNF-α, interferon-γ, interleukin- 17, and interleukin-23.
The oral PDE-4 inhibitor apremilast has been approved by the FDA for the treatment of moderate-to-severe psoriasis, but there is currently no approved topical PDE-4 inhibitor for psoriasis patients.
The newly published phase IIb trial of topical roflumilast (Acrutis Biotherapeutics) included 331 patients with plaque psoriasis randomized to treatment with roflumilast 0.3% cream 9 (n=109), roflumilast 0.15% cream (n=113) or placebo vehicle cream (n=109). once daily for 12 weeks.
All patients were ≥18 years of age with plaque psoriasis for at least 6 months and a score of at least 2 on the investigator global assessment (IGA) 5-point psoriasis severity score.
The primary study endpoint was IGA status of 0 or 1, indicating clear or nearly clear skin at week 6. The IGA assesses plaque thickening, scaling and erythemia, rating from 0 (clear) and 1 (almost clear to 4 (severe).
Among the main study findings:
- At treatment week 6, 28%, 23% and 8% of patients in the roflumilast 0.3%, 0.15% and placebo groups, respectively, had achieved clear or almost clear skin (P<0.001 and P=0.004 vs. vehicle for roflumilast 0.3% and 0.15%, respectively).
- At week 6, an IGA score indicating clear or almost clear plus a 2-grade improvement in the intertriginous-area IGA score occurred in 73% of the patients in the roflumilast 0.3% group, 44% of those in the roflumilast 0.15% group, and 29% of those in the vehicle group.
- Mean baseline Psoriasis Area Severity Index (PASI) scores were 7.7 in the roflumilast 0.3% group, 8.0 in the roflumilast 0.15% group, and 7.6 in the vehicle group; the mean change from baseline at week 6 was −50.0%, −49.0%, and −17.8%, respectively.
Study limitations included its short duration and the small number of patients with intertriginous psoriasis (approximately 15% of the participants).
The researchers concluded that “longer and larger trials are necessary to determine the effectiveness and safety of roflumilast.”
-
Roflumilast cream, which is a topical version of a phosphodiesterase type 4 (PDE-4) inhibitor, was significantly more effective than placebo for reducing psoriasis severity in a 12-week, phase II clinical trial.
-
At 6 weeks, more patients with chronic plaque psoriasis treated with once-daily roflumilast cream at 0.3% and 0.15% strength achieved clear or almost clear skin than patients treated with a placebo vehicle cream: 28% (0.3%) and 23% (0.15%) versus 8%.
Salynn Boyles, Contributing Writer, BreakingMED™
This study was funded by Arcutis Biotherapeutics.
Lead researcher Mark Lebwohl reported receiving grant support from Ortho Dermatologics, UCB, AbbVie, Amgen, Eli Lilly, Incyte, and Janssen Research and Development, grant support and consulting fees from Pfizer, Dermavant Sciences, LEO Pharma, Boehringer Ingelheim, and Arcutis Biotherapeutics, and consulting fees from Allergan, Almirall, Avotres Therapeutics, BirchBioMed, Bristol- Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, EMD Serono, Evelo Biosciences, Inozyme Pharma, Meiji Seika Pharma, Menlo Therapeutics, Mitsubishi Pharma, NeuroDerm, Promius Pharma–Dr. Reddy’s Laboratories, Theravance Biopharma, Verrica Pharmaceuticals, Aditum Bio, BMD Skincare,and Kyowa Kirin.
Prinicipal researcher Howard Welgus reported being an employee of Arcutis Biotherapeutics and holding a pending patent on a formulation for improving roflumilast skin-penetration lag time.
Several other study authors were also employed by Arcutis Biotherapeutics.
Cat ID: 10
Topic ID: 75,10,730,10,105,192,923,925