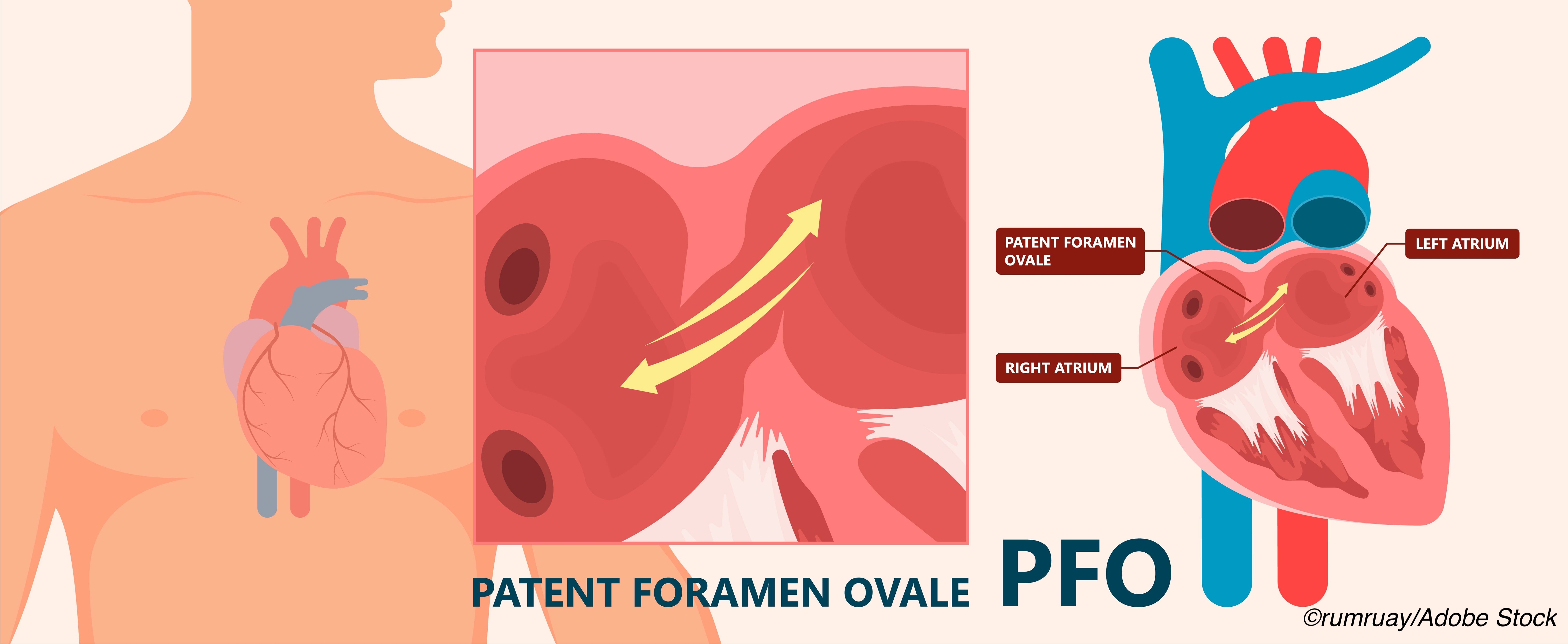
Closure of patent foramen ovale (PFO) appeared to reduced migraine frequency, an analysis that combined patient-level data from two prospective clinical trials suggested.
“This pooled analysis of patient-level data demonstrates that PFO closure was safe and significantly reduced the mean number of monthly migraine days and monthly migraine attacks, and resulted in a greater number of subjects who experienced complete migraine cessation,” wrote Mohammad Mojadidi, MD, of Virginia Commonwealth University in Richmond, and co-authors in Journal of the American College of Cardiology.
The analysis pooled data from two trials of episodic migraine patients with PFO that individually failed to meet their defined efficacy endpoints: PREMIUM (n=230, 89% female, mean age 43, 66% with aura) and PRIMA (n=107, 84% female, mean age 43, 99% with aura). Both trials suggested participants with aura had a better response to closure.
Mojadidi and colleagues defined four outcomes: mean reduction in monthly migraine days, mean reduction in number of monthly migraine attacks, complete migraine cessation, and responder rate (defined as patients with 50% or more reduction in number of attacks per month).
In the pooled cohort, 176 patients were randomized to device closure and 161 to medical treatment. Eventually, 275 people received devices and were counted in the safety analysis.
At 10- to 12-month follow-up:
- Mean reduction of monthly migraine days was 3.1 vs 1.9 days, favoring closure, P=0.02.
- Mean reduction of monthly migraine attacks was 2.0 vs 1.4, favoring closure, P=0.01.
- Number of people who experienced complete cessation of migraine was 14 (9%) versus 1 (0.7%), favoring closure, P<0.001.
- Responder rate was not significantly different between closure and medical groups.
Results were driven largely by migraine patients who had frequent aura.
PRIMA and PREMIUM failed to meet their primary endpoints in part because of their small sizes and limited statistical power, noted Zubair Ahmed, MD, of the Cleveland Clinic in Ohio and Robert Sommer, MD, of Columbia University in New York City, in an accompanying editorial.
In this analysis, “the investigators were able to demonstrate benefit of PFO closure in the migraine population for the first time,” they observed. “Moreover, the investigators defined a population of patients who may benefit most from PFO closure: those with migraine with frequent aura, suggesting that these may be different physiologically than other migraine subtypes.”
PFO allows blood to bypass the lungs and return directly to systemic circulation via the left atrium and ventricle. A thrombus, if present, is not filtered by the lungs and may cause tissue ischemia including stroke. PFO closure for stroke prevention is considered for patients with cryptogenic stroke of presumed embolic origin with passage through the PFO, particularly people age 60 or younger without vascular risk factors.
Up to 25% of the general population may have PFO and an even higher rate, 30-50%, is seen in people with migraine with aura, the researchers noted, though not all people with PFO have migraine.
“If closure of PFOs provides real headache relief in some patients, the mechanism must involve the right-to-left passage of systemic venous blood, as in PFO-related stroke,” Ahmed and Sommer observed. “Some blood component, which would normally be eliminated or reduced on passage through the pulmonary vasculature, could reach the cerebral circulation via the PFO in supranormal concentrations and act as a trigger for migraine activity in patients with susceptible brains.”
PRIMA studied migraine with aura; results published in 2016 failed to show reduction in monthly migraine days (the primary endpoint) in the group receiving closure.
In 2017, PREMIUM authors reported their 1-year double-blind randomized trial that included medical therapy with sham right heart catheterization versus medical therapy with PFO closure for migraine with and without aura. There was no difference in responder rate between groups (the primary endpoint), though the closure group did have a greater reduction in headache days.
“As there remains no verified way to distinguish between ’causal’ and ’incidental’ PFO, these and other migraine/PFO trial data could have been critically influenced by the unknown ratio of causal to incidental PFOs,” Ahmed and Sommer added.
Mojadidi and colleagues’ pooled population had 8-10 migraine days and 4-5 attacks monthly at baseline. They designated reduction of monthly migraine days, assessed at 10-12 months after randomization, as the primary endpoint.
Subgroup analysis of patients with and without aura found those with aura in >50% of migraine attacks who had PFO closure had even greater reductions in monthly migraine days (4.3) compared with controls, as well as greater complete headache cessation. Patients without aura who had PFO closure did not have a significant reduction in migraine days or cessation.
Safety outcomes included <1% occurrence of procedure-related events (nine events total, including access site bleeding, arm phlebitis, hematoma, hypotension, tachycardia, and vasovagal episode). Possible device-related outcomes were also <1% (four total, including fatigue, nonsustained atrial fibrillation, and syncope).
“Designing a randomized trial to prove the PFO migraine mechanism beyond a reasonable doubt will require an inclusion or screening criterion that can reliably determine the causal or incidental nature of the PFO,” the editorialists noted. “Only then will the benefit of PFO closure therapy be truly knowable (the inclusion of only causal PFO would likely also significantly increase the rate of beneficial response to the therapy).”
The planned 2021 RELIEF Migraine trial that will use responsiveness to P2Y12 platelet inhibition as an inclusion criterion is an example, they added: “Patients will need to demonstrate a 50% reduction in monthly migraine days with documented P2Y12 receptor inhibition.”
Limitations include differences between the two studies: PRIMA was unblinded and may have introduced a placebo-effect bias. In addition, PRIMA involved migraine patients with aura while PREMIUM had a mixed population. There was no distinction between incidental and causal PFOs in either study.
-
Closure of patent foramen ovale (PFO) appeared to reduce migraine frequency, an analysis that combined patient-level data from two prospective clinical trials suggested.
-
The analysis pooled data from two trials that individually failed to meet their defined endpoints but suggested that participants who had migraine with aura had a better response to closure.
Paul Smyth, MD, Contributing Writer, BreakingMED™
Mojadidi had no relationships relevant to the contents of this paper to disclose.
Ahmed has received consulting fees from Eli Lilly, Amgen, Abb- Vie, and ElectroCore; has served on advisory boards for Amgen and Supernus; has served as a speaker for AbbVie; and has received funding for an investigator-initiated trial from Teva and Eli Lilly. Sommer has reported that he has no relationships relevant to the contents of this paper to disclose.
Cat ID: 130
Topic ID: 82,130,730,914,130,35,192,925