To help clinicians determine which men with recurrent prostate cancer after surgery will benefit from the addition of hormone therapy to salvage radiotherapy (sRT), researchers validated the Decipher genomic classifier, demonstrating that it was independently associated with the risk of distant metastases (DM), prostate cancer-specific mortality (PCSM), and overall survival (OS).
Importantly, these results suggest that not all men with biochemically recurrent prostate cancer will derive the same benefits from the addition of hormone therapy to sRT.
“To date and to our knowledge, no multigene expression biomarker has been rigorously validated as a prognostic or predictive test in patients with prostate cancer who are receiving radical therapy,” Felix Y. Feng, MD, of the Helen Diller Family Comprehensive Cancer Center, San Francisco, and colleagues observed. “[These] findings add to the evidence base supporting the use of the GC to guide shared decision-making after RP.”
When used to analyze radical prostatectomy (RP) specimens from men with recurrent prostate cancer, the 22-gene GC was associated with a 17% increased risk of distant metastases (HR 1.17, 95% CI, 1.05-1.32; P=0.006), and was also independently associated with a 39% greater likelihood of PCSM (HR 1.39, 95% CI, 1.20-1.63, P<0.001), as well as a 17% worse prognosis in terms of OS after adjusting for standard clinical and pathological variables (HR 1.17, 95% CI 1.06-1.29, P=0.002), Feng colleagues reported in JAMA Oncology.
For the randomized, phase III, NRG/RTOG 9601 study, Feng and colleagues enrolled patients receiving sRT to additional treatment with the antiandrogen bicalutamide (150 mg, twice daily given for 2 years) or placebo.
“Eligible patients were required to have recurrent disease after RP with a PSA (prostate-specific antigen) of 0.2 to 4.0 ng/mL, pathologic T3 disease (tumor spread beyond the prostate) or T2 disease (tumor contained within the prostate) with a positive surgical margin and no evidence of nodal or metastatic disease,” they noted.
sRT was delivered at doses of 64.8 Gy/1.8 Gy per fraction using 2- or 3-dimensional techniques. At a median follow-up of 13 years, RP specimens were reviewed centrally, and RNA was analyzed from the highest-grade tumor available in 2019. GC scores were generated from 486 of 760 patients, while samples from 352 men passed a microarray quality control test and made up the final cohort for analysis.
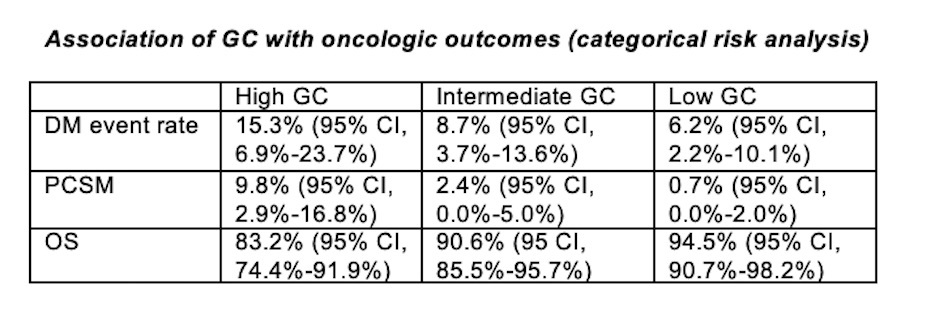
In this final cohort, 42% of men had a low GC, 38% had an intermediate GC, and 20% had a high GC, according to the authors, who analyzed GC both by the 3-tier risk group as well as a continuous variable on a scale from 0 to 1. Upon univariate analysis, the GC significantly stratified the risk of multiple oncologic outcomes.
“The difference between GC low risk and higher risk groups for the prespecified primary end point of DM was large and statistically significant,” the authors emphasized.
Specifically, men with an intermediate and high GS score had an almost 90% greater risk of developing distant disease compared with men with a low GC scores, they pointed out. On the other hand, the interaction between GC scores and the hormone’s effect on DM, PCSM, and OS was not statistically significant.
Nevertheless, “the estimated absolute benefits in DM, PCSM, and OS observed with hormone therapy were different by GC risk groups,” Feng and colleagues observed.
For example, at 12-year follow-up, the benefit of bicalutamide was roughly 3-fold greater in men with intermediate and high GC scores than in those with a low GC score, at a DM rate of 5% vs 15.7%, respectively; a PCSM of 4.5% vs 11.8%; and an OS of 2.4% vs 8.9% for low versus intermediate/high GC risk categories, respectively.
“The GC score was [also] prognostic in the subset of patients treated with earlier sRT when PSA was less than the median entry value (0.7 ng/mL),” Feng and colleagues observed.
Men with a higher GC score had an 11.2% improvement in their 12-year risk of DM and a 4.6% improvement in OS with the addition of hormone therapy, compared with only a 0.4% benefit for men in the low GC risk group. Furthermore, the absolute effect of hormone therapy on 12-year PCSM rates for men in the low GC risk category was 1.0%, compared with 8.4% for men with higher GC scores, and on OS, was -7.8% vs 4.6%, respectively.
As the authors pointed out, a prognostic biomarker such as the Decipher GC analyzed in this study can help identify patients at low risk of recurrence as well as those who are unlikely to benefit from treatment intensification.
For example, patients in the study with lower GC scores only had a 2.4% absolute improvement in their 12-year OS rates compared with an 8.9% improvement in patients with higher GC scores.
“For some patients, the risk of toxic effects, cost, and possible effects on quality of life from long-term hormone therapy may erode further OS benefit,” the authors suggested.
“The results of this and other studies strongly suggest that not all men with biochemically recurrent prostate cancer after surgery derive equal absolute benefits from the addition of hormone therapy to sRT,” they concluded.
Commenting on the findings, Sean E. McGuire, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, pointed out that thus far, clinical decision-making between men with prostate cancer and their physician has relied primarily on traditional clinicopathologic features such as Gleason score and PSA levels.
“However, at best, these measures serve as incomplete surrogates for the actual underlying biology driving the behavior of the disease in each patient,” he noted.
As such, McGuire called the report by Feng and colleagues a “hugely important milestone,” in that it provides what he calls “the first level-1 validation” of the performance of the Decipher GC in a prospective, randomized, double-blind, phase III trial.
“In the end, this study recalls the landmark study by Paik and colleagues, and its effect should lead to reconsideration of ASCO guidelines that recommend the use of Decipher GC testing on the basis of the strength of the evidence and its adoption into routine clinical use should become commonplace, as it is in breast cancer management. The authors are to be congratulated for this long-awaited achievement in prostate cancer,” McGuire concluded.
-
The Decipher genomic classifier was independently associated with future risk of distant metastases, prostate cancer-specific mortality and overall survival in men with recurrent prostate cancer.
-
The same multigene biomarker can help select out men who are less likely to benefit from additional hormonal therapy following salvage radiotherapy and who thus may be spared the potential toxicity and cost of long-term androgen suppression.
Pam Harrison, Contributing Writer, BreakingMED™
The trial was sponsored by the National Cancer Institute.
Feng reported receiving personal fees from Decipher, PFS Genomics, Diagnostics, Astellas, Janssen, Roivant, Myovant, Genentech, Janssen Oncology, Sanofi, and Bayer as well as grants from Zenith Epigenetics. He also has ownership interests in PFS Genomics.
Cat ID: 25
Topic ID: 78,25,496,730,25,192,73,925


