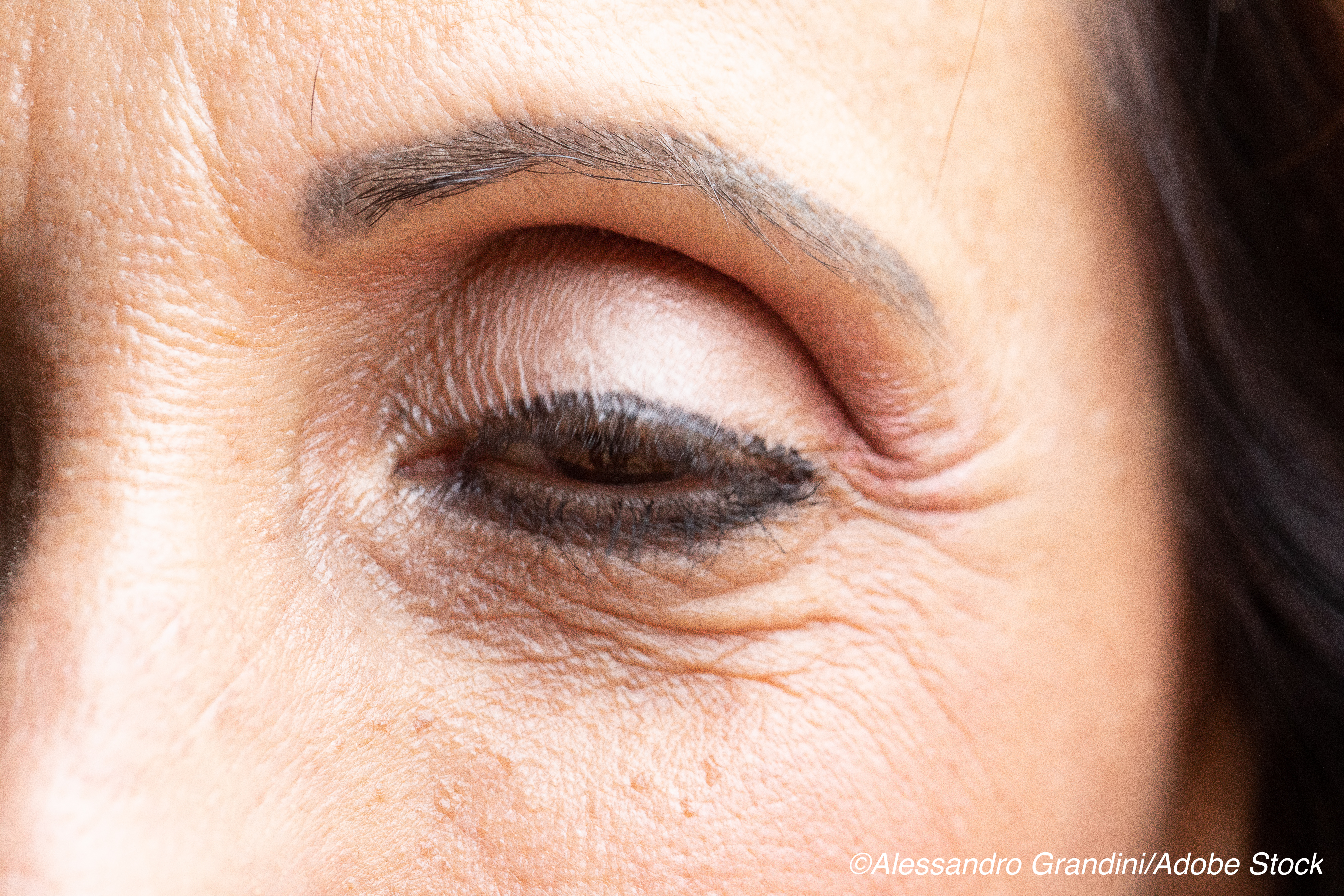
Results from those trials demonstrated that oxymetazoline, 0.1%, was associated with greater visual field improvement and upper eyelid elevation in patients with ptosis.
A study based on the two trials was published in JAMA Ophthalmology.
According to the authors, led by Charles B. Slonim, MD, Department of Ophthalmology, University of South Florida Morsani College of Medicine, Tampa, ptosis not only leads to visual field defects, but can also lead to elevated levels of anxiety, depression, and appearance-related distress. It is a fairly common disorder, particularly among elderly patients.
Treatment is currently limited to surgical interventions. However, there are risks involved with surgery, including asymmetry, overcorrection/undercorrection, bleeding, lagophthalmos, and infection.
Oxymetazoline nasal spray — known by its trade name Afrin — was approved by the FDA more than 50 years ago as a decongestant. Oxymetazoline is also able to elevate the eyelid by activating α-adrenergic receptors in the superior tarsal (Muller) muscle. In July, the FDA approved a new formulation — oxymetazoline hydrochloride, 0.1%, ophthalmic solution — for the treatment of acquired ptosis, based on the results of randomized, double masked, placebo-controlled, multicenter phase 3 clinical trials ( RVL-1201-201 [NCT02436759] and RVL-1201- 202 [NCT03565887]).
The two studies included 304 patients with acquired ptosis, 203 of whom received oxymetazoline, 0.1%, and the remainer of whom received a vehicle control, as a single drop per eye, once daily, for 42 days.
In their pooled analysis of the two studies, Slonim and colleagues found that oxymetazoline, 0.1%, was associated with a significant increase of between 4 and 5 points as seen on the Leicester Peripheral Field Test compared to the vehicle after administration at 1 and 14 days (day 1: 5.9 [6.4] vs 1.8 [4.1]; mean difference, 4.07 [95% CI, 2.74-5.39], and day 14: 7.1 [5.9] vs 2.4 [5.5]; mean difference, 4.74 [95% CI, 3.43-6.04]).
The authors also observed that oxymetazoline, 0.1%, was associated with a significant increase in upper eyelid height compared to vehicle, with a mean difference of 0.47 mm at day 1, and 0.67 mm at day 14.
As for safety, there were 129 treatment-emergent adverse events (TEAEs) in 63 participants (31.0%) receiving oxymetazoline, 0.1%, and 73 TEAEs in 36 participants (35.6%) receiving vehicle. The majority of TEAEs were of mild intensity among those patients receiving oxymetazoline, 0.1%, although these patients had higher rates of punctate keratitis (oxymetazoline, 0.1%: 11 of 203 [5.4%]; vehicle: 3 of 101 [3.0%]), blurred vision (oxymetazoline, 0.1%: 7 of 203 [3.4%]; vehicle: 0), and conjunctival hyperemia (oxymetazoline, 0.1%: 6 of 203 [3.0%]; vehicle: 1 of 101 [1.0%]).
“These clinical study results support the efficacy and safety of oxymetazoline, 0.1%, for the treatment of acquired ptosis, a potentially important finding given the lack of other approved pharmacologic options for this condition, as well as the barriers to and potential risks associated with surgery,” wrote Slonim and colleagues.
In a commentary accompanying the study, Elizabeth A. Bradley, MD, MHS and David J. Bradley, MD, PhD, ScM, both of the Mayo Clinic, , wrote that the obvious clinical application of oxymetazoline, 0.1%, is for patients who have a minimal amount of ptosis, but who have no guarantee that perfect results will be achieved with a surgical intervention.
“If such a patient could use a daily eye drop to alleviate ptosis and avoid surgery, both patient and surgeon would likely embrace the therapy,” they wrote.
They added that further study is needed to demonstrate the effect oxymetazoline, 0.1% has on health-related quality of life, as well as costs, durable treatment effect, and adverse effects with long-term use.
“Even with such future studies, the modest improvements in visual field and eyelid height reported in this study suggest that the drug may be most useful for managing mild ptosis or for more substantial ptosis in which surgical correction is not advised because of ocular or medical comorbidities,” they concluded. “For that group of patients, the “new trick” offered by this “old dog” could be very appealing.”
-
Administration of oxymetazoline, 0.1% to patients with ptosis led to improved vision outcomes and was well tolerated, according to results from a pair of phase III trials.
-
Patients treated with oxymetazoline, 0.1% achieved greater visual field improvement and upper eye elevation compared to patients who received a vehicle control.
Michael Bassett, Contributing Writer, BreakingMED™
Slonim reports personal fees from RevitaLid during the conduct of the study and outside the submitted work and is a paid consultant to RVL Pharmaceuticals, a subsidiary of Osmotica Pharmaceuticals.
The commentary authors had no disclosures.
Cat ID: 240
Topic ID: 92,240,282,494,192,255,925,240


