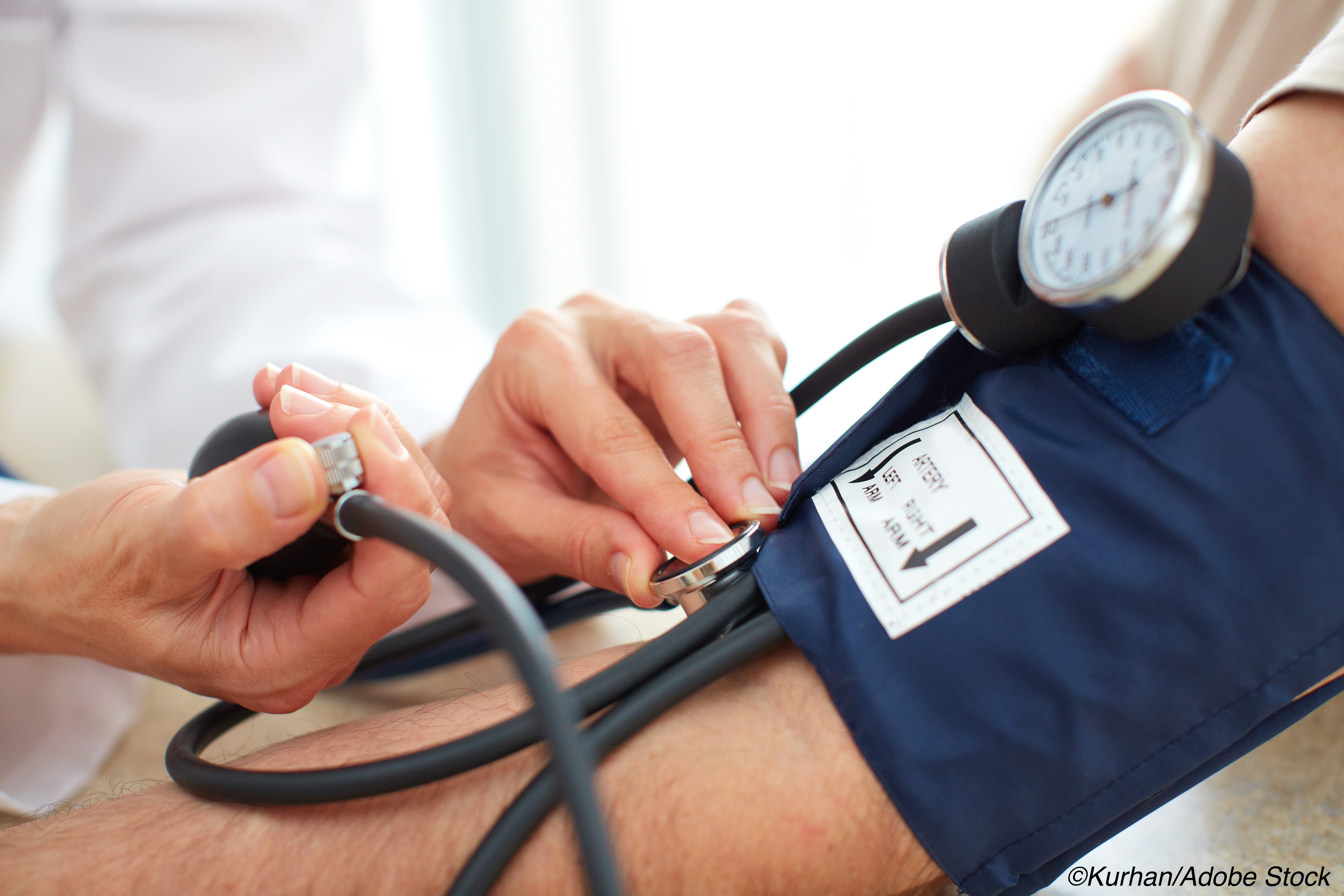
Office-based blood pressure (BPs) measurements to screen for adult hypertension are still a go, but BP monitoring should also be done outside of the clinic to confirm the diagnosis before starting treatment, according to the U.S. Preventive Services Task Force (USPSTF).
In a re-affirmation of its 2015 “A” grade recommendation, the task force “found convincing evidence that screening for hypertension with office blood pressure measurement and treatment of hypertension in adults substantially reduce the incidence of cardiovascular [CV] events,” wrote Alex H. Krist, MD, MPH, Virginia Commonwealth University in Richmond, and co-authors.
“Using a reaffirmation deliberation process, the USPSTF concludes with high certainty that screening for hypertension in adults has substantial net benefit,” they explained in the Journal of the American Medical Association (JAMA).
Krist and co-authors outlined a two-step process in the re-upped recommendation:
- Screen by measuring BP with an office-based BP measurement (OBPM).
- Confirm by taking BP measurements outside of the clinical setting to confirm a hypertension diagnosis pre-treatment.
In terms of methods of outpatient BP tracking, the task force supported ambulatory BP monitoring (ABPM) where “patients wear a programmed portable device that automatically takes blood pressure measurements, typically in 20- to 30-minute intervals over 12 to 24 hours while patients go about their normal activities or are sleeping.” Another option is home BP monitoring where “patients measure their own blood pressure at home with an automated device,” with measurements taken one to two times a day or week.
In either case, BP data should be taken at the “brachial artery (upper arm) with a validated and accurate device in a seated position after 5 minutes of rest,” they stressed.
Some people are due for more frequent BP tracking than others, according to the task force, specifically annual screening those ages ≥40 at increased risk for hypertension, including Blacks, people with high-normal BP, or those who are overweight or obese. However, screening every 3 to 5 years is acceptable in those ages 18 to 39 who are not at increased risk for hypertension and who had a previous normal BP reading.
As for potential harms of BP monitoring, Krist’s group said that there were “few major harms” linked with hypertension screening and treatment in clinical settings.
A series of accompanying editorials offered a cohesive message on the recommendation: It’s all good, but it’s certainly not enough.
The recommendation offers “an appropriate floor condition, setting the minimum standard expected for BP measurement in the diagnosis and confirmation of elevated BP levels consistent with hypertension,” noted Donald M. Lloyd Jones, MD, ScM, of Northwestern University Feinberg School of Medicine in Chicago, and co-authors, in JAMA.
Eric D. Peterson, MD, MPH of UT Southwestern in Dallas, and co-authors placed the guideline in the context of a public health crisis. The “Covid-19 pandemic has also demonstrated the value of community partnerships in improving health outcomes,” they explained in JAMA. “Preventing hypertension-related adverse outcomes should not solely be the responsibility of individual patients and health care workers… critical component of a national strategy will be to optimize patient care for hypertension and ensure that the places where people live, learn, work, and play support hypertension control.”
In JAMA Network Open, Keith C. Ferdinand, MD, of the Tulane Heart and Vascular Institute in New Orleans, and Angela L. Brown, MD, of Washington University School of Medicine in St Louis, also cited the pandemic as bringing a shift to the hypertension care paradigm: “The use of self-measured BP supported through telemedicine by health care practitioners will likely become increasingly important for hypertension control as the pandemic continues and in future care models, thus potentially facilitating patient centric care as patients become more aware of their disease process.”
Also in JAMA Network Open, Daichi Shimbo, MD, Columbia University Irving Medical Center in New York City, and co-authors, conceded that “[e]mphasizing the importance of out-of-office BP monitoring is meaningful.”
The experts stressed that the recommendation has limitations on clinical and societal levels.
Lloyd Jones’ group pointed out that the USPSTF guidance is not in line with those from other groups, such as the American College of Cardiology/American Heart Association and the European Society of Cardiology/European Society of Hypertension, all of which call for “more intensive screening and confirmation strategies.” The American Academy of Family Physicians supported the 2015 USPSTF recommendation.
Peterson and co-authors noted that “Measuring blood pressure can be used to identify hypertension, but in isolation, does not improve blood pressure control,” so extra measures, such as home telemonitoring need to be broadly deployed.
Lloyd Jones and co-authors emphasized that “individuals from socially deprived backgrounds, who are at high risk for hypertension, are less likely to have access to or to participate in office-based hypertension screening.”
Ferdinand and Brown questioned if the “recommended 2-stage screening method will further worsen or will decrease racial/ethnic disparities in BP control…the new USPSTF…recommendations are a unique approach with the potential to have a positive impact on hypertension control and outcomes in Black, Hispanic/Latinx, and other minority populations. Nevertheless, issues related to access to and implementation of effective BP recognition and control measures among various populations must be addressed.”
Shimbo’s group criticized the 2021 recommendation for not addressing “the poor implementation of ABPM in clinical practice,” citing lack of widespread availability of APBM, not to mention barriers for getting it up and running, including staff training and time constraints. In a JAMA Patient Page, Jill Jin, MD, MPH, of Northwestern Medicine in Chicago noted that APBM can “cause some discomfort for patients,” while the evidence report supporting the recommendation stated that APBM “was associated with temporary sleep disturbance and bruising.”
The evidence report by Janelle M. Guirguis-Blake, MD, of Kaiser Permanente Evidence-based Practice Center at the University of Washington in Seattle, and co-authors included 52 studies with more than 200,000 patients.
Jin explained that “[d]ifferent organizations use slightly different definitions for high blood pressure: the cutoff values for what is considered ’high’ range from higher than 130/80 mm Hg to higher than 140/90 mm Hg. For the purposes of this recommendation statement, studies using any cutoff to define hypertension were reviewed.”
Guirguis-Blake and co-authors reported that one cluster randomized clinical trial RCT with 140,642 patients looked at a multicomponent intervention that included hypertension screening. That study reported fewer annual CV-related hospital admissions for CV disease in the intervention group versus the control group for a difference of 3.02 per 1,000 people (rate ratio 0.91, 95% CI 0.86-0.97).
Additionally, a meta-analysis of 15 studies (n=11,309) of initial office-based BP screening showed a pooled sensitivity of 0.54 (95% CI 0.37-0.70) and a specificity of 0.90 (95% CI 0.84-0.95), but with considerable clinical and statistical heterogeneity, the authors cautioned.
Guirguis-Blake’s group also found that 18 studies of various confirmatory BP measurement modalities were heterogeneous, while 13 studies “suggested that screening was associated with no decrement in quality of life or psychological distress; evidence on absenteeism was mixed.”
Another meta-analysis of eight office-based confirmation studies (n=53,183) demonstrated a pooled sensitivity of 0.80 (95% CI 0.68-0.88) and a specificity of 0.55 (95% CI 0.42-0.66), while a meta-analysis of four home-based confirmation studies (n=1,001) turned in a pooled sensitivity of 0.84 (95% CI, 0.76-0.90) and a specificity of 0.60 (95% CI 0.48-0.71).
The authors ultimately concluded that “[s]creening using office-based blood pressure measurement had major accuracy limitations, including misdiagnosis; however, direct harms of measurement were minimal.”
However, both Guirguis-Blake’s group and co-authors Krist’s group acknowledged the challenges presented by white coat and/or masked hypertension.
Guirguis-Blake and colleagues noted that any “hypertension screening algorithm using measurement modalities other than ABPM alone will incur a considerable number of missed cases of masked hypertension as well as treatment of white coat hypertension. However, the clinical significance of the poor accuracy of OBPM is largely unknown,” although delays in the identifying and treating masked hypertension is problematic as is unnecessary treatment for white coat hypertension.
Krist’s group explained that the “association of masked hypertension and white coat hypertension with increased cardiovascular risk has been well documented, it is unclear whether treatment of either of these types of hypertension improves health outcomes. The USPSTF considers this a critical evidence gap.”
-
Adults should undergo screening for hypertension with office blood pressure (BP) measurement, per a re-affirmed U.S. Preventive Services Task Force (USPSTF) recommendation.
-
The USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment for hypertension.
Shalmali Pal, Contributing Writer, BreakingMED™
The USPSTF is funded by the Agency for Healthcare Research and Quality (AHRQ). The evidnce report was funded by AHRQ.
USPSTF members reported travel reimbursement and an honorarium for participating in USPSTF meetings.
Peterson reported support from Bristol Myers Squibb and a relationship with Livongo.
Lloyd-Jones, Guirguis-Blake, and co-authors and reported no relationships relevant to the contents of this paper to disclose.
Ferdinand reported relationships with Medtronic, Amgen, and Novartis. Brown reported support from the NIH and Medtronic/Washington University School of Medicine.
Shimbo and a co-author reported support from the National Heart, Lung, and Blood Institute.
Jin reported serving as JAMA associate editor.
Cat ID: 6
Topic ID: 74,6,730,6,192,151,925


