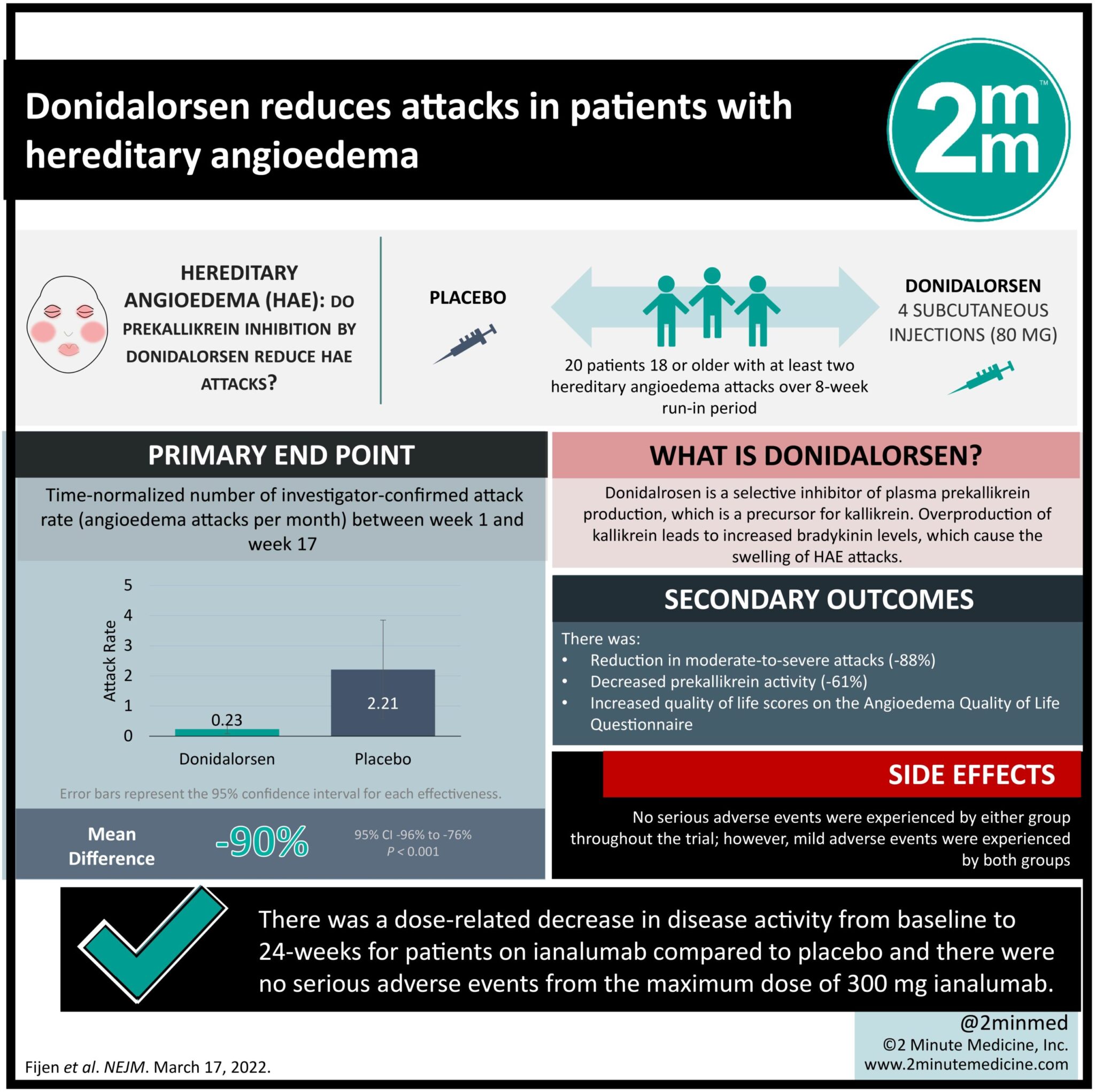
- Patients with hereditary angioedema receiving donidalorsen showed reduced rates of angioedema attacks compared to patients receiving placebo treatment.
- Donidalorsen shows an acceptable safety profile after four doses.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Patients with hereditary angioedema experience unpredictable swellings that could potentially be fatal. Donidalorsen is an antisense oligonucleotide treatment that inhibits the production of plasma prekallikrein, a key contributor to excessive vascular permeability. In this phase 2 trial, patients with hereditary angioedema with C1 deficiency were randomized in a 2:1 ratio to either receive four doses of donidalorsen or placebo treatment. The primary endpoint was the number of confirmed angioedema attacks per month between experimental groups. Secondary endpoints analyzed the number of moderate-to-severe attacks, prekallikrein levels, and impact on quality of life measures. The study found that at a 17-week follow-up, patients receiving donidalorsen experienced a lower rate of investigator-confirmed angioedema attacks per month compared to patients receiving placebo. This group also experienced an improvement in quality of life measures and a reduction in prekallikrein activity. Safety data demonstrate similar rates of adverse events in both groups with the most common event being headache and nausea. Taken together, this study supports the use of donidalorsen as a treatment for patients with hereditary angioedema and warrants further investigation on its safety and efficacy. The study is limited by its very small sample size and selection of patients with C1 inhibitor deficiency, limiting the generalizability of these results to all patients with hereditary angioedema.
Click to read the study in NEJM
Relevant Reading: Antisense Inhibition of Prekallikrein to Control Hereditary Angioedema
In-Depth [randomized controlled trial]: In this small phase 2 randomized control trial, 20 patients with laboratory-confirmed hereditary angioedema with a C1 inhibitor deficiency (activity < 40%) were randomized in a 2:1 ratio to either receive 80 mg of donidalorsen or placebo treatment by subcutaneous injection on a schedule of 4 doses each spaced 4 weeks apart. Patients were then tracked for frequency and severity of investigator-confirmed angioedema attacks throughout the 17-week study. Secondary endpoints analyzed differences in frequency of moderate-to-severe attacks, attacks in the second half of the study, prekallikrein levels, and impact on quality of life measures. Differences in rates were analyzed using a Poisson regression model. The study found that the 14 patients who received donidalorsen had a mean monthly rate of 0.23 investigator-confirmed angioedema attacks (95% confidence interval [CI], 0.08 – 0.39), which was significantly lower than the 8 patients in the placebo group who had 2.21 (95% CI, 0.58 – 3.85). This represents a -90% mean difference between groups (95% CI, -96 to -76; P<0.001). With respect to secondary outcomes, patients receiving donidalorsen also demonstrate a reduction in moderate-to-severe attacks (-88%), decreased prekallikrein activity (-61%), and increased quality of life scores on the Angioedema Quality of Life Questionnaire. No serious adverse events were experienced by either group throughout the trial; however, mild adverse events were experienced by both groups with headache and nausea being the most common. Overall, the results of this small trial were able to support the use of donidalorsen in patients with hereditary angioedema and confirmed C1 inhibitor deficiency. Continued investigation for safety and efficacy in larger and longer trials is warranted.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.