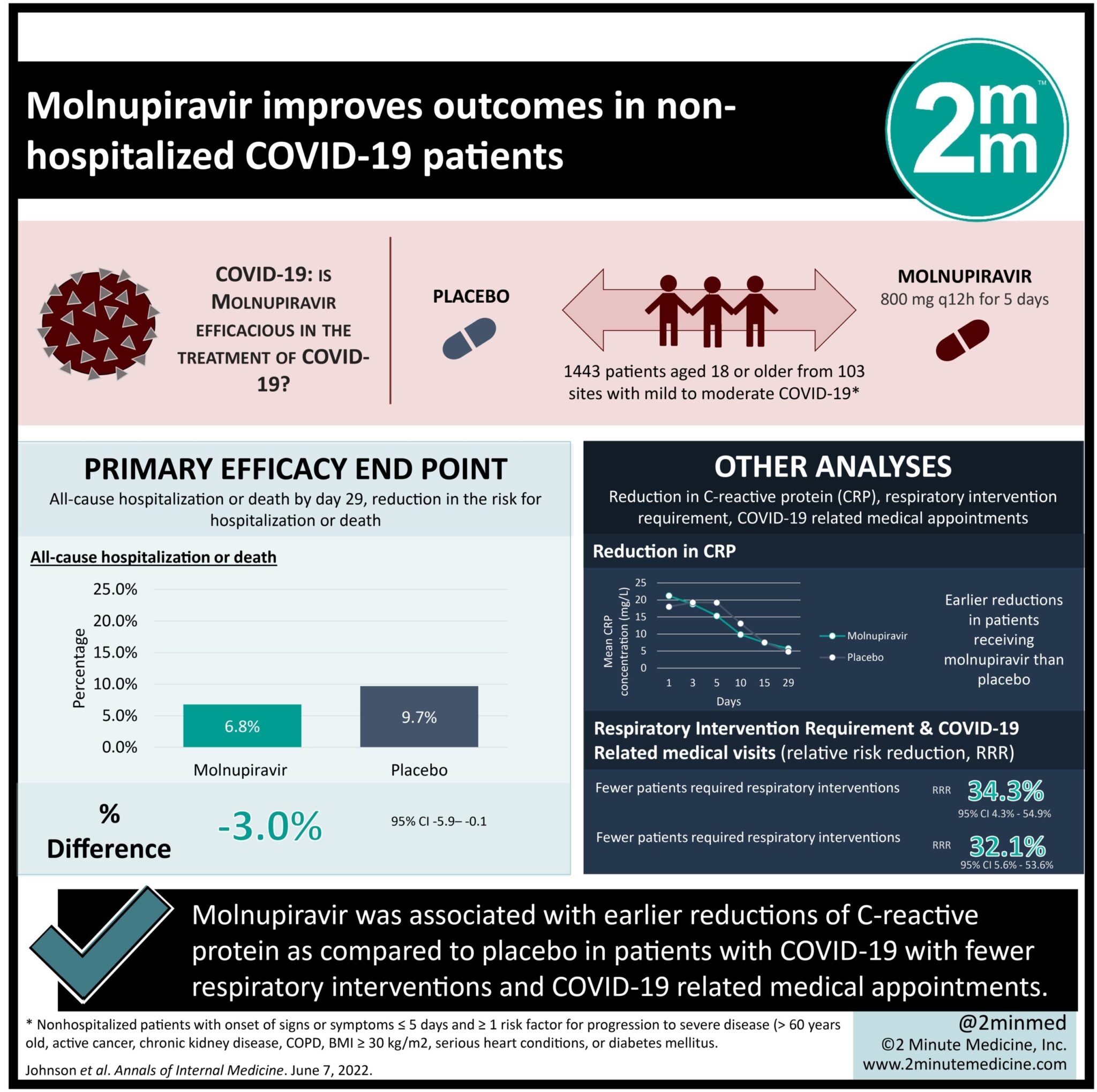
1. In coronavirus disease 2019 (COVID-19) patients, molnupiravir was associated with earlier and larger reductions of C-reactive protein (CRP) as compared to placebo.
2. Participants who received molnupiravir required fewer respiratory interventions and COVID-19-related medical appointments.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Several approved monoclonal antibodies (mAB) have been shown to reduce the risk of hospitalization and severe illness in COVID-19 patients. However, many of the existing options for COVID-19 intervention require injections in-hospital or have been found to be ineffective against the Omicron (B.1.1.529) variant. Molnupiravir is an orally available mAB treatment option that has been shown to have broad-spectrum activity against SARS-CoV-2 variants of concern. Patients 18 or older with confirmed mild to moderate COVID-19 were randomly assigned to receive molnupiravir 800 mg or placebo every 12 hours for 5 days. Those in the treatment group saw an earlier and larger reduction in CRP at all study visits than placebo. Molnupiravir-treated participants also had an improvement in SpO2 values as early as three days following treatment, compared to 10 days in the placebo group. The requirement for respiratory interventions was significantly reduced for the treatment group. Finally, acute care visits for COVID-19-related symptoms were significantly lower in the treatment group than in the placebo group. A limitation is that longer-term benefits of molnupiravir therapy were not studied as the endpoint was 29 days after treatment and there could potentially be long-lasting protective effects for patients.
Click to read the study in AIM
In-Depth [randomized controlled trial]: The MOVe-OUT phase three trial examined the effect of molnupiravir on inflammatory biomarkers, respiratory intervention requirements, and medical care usage in COVID-19 patients. In total, 1,433 participants from 103 sites were randomly assigned to either receive molnupiravir (n=710) or a placebo control (n=701). Further, 152 patients in the treatment group and 151 in the placebo group received systemic corticosteroids before or during the study treatment period. Molnupiravir-treated participants had earlier and larger reductions in CRP after treatment on days three, five, 10, 15, and 29. However, these findings were not statistically significant compared to the placebo. When changes in SpO2 were examined, the treatment saw immediate improvements starting on day three, compared to day 10 for the placebo group. In the modified intention-to-treat (MITT) group, fewer patients in the treatment group required the use of respiratory interventions (Relative risk reduction [RRR] 34.3%; 95% Confidence Interval [CI], 4.3% to 54.9%). The respiratory interventions examined were conventional oxygen therapy, high-flow heated and humidified device, non-invasive mechanical ventilation, and invasive mechanical ventilation. Participants who had a COVID-19-related visit were lower in the treatment group (6.6%) compared to the placebo group (10%) (RRR 32.1%; 95% CI, 5.6% to 53.6%). 48 participants (6.8%) in the molnupiravir-treatment group and 67 participants (9.6%) in the placebo group were hospitalized during the study period. The findings suggest that molnupiravir may benefit non-hospitalized, mild to moderate COVID-19 patients by reducing health care visits/hospitalizations, and costs while alleviating symptoms.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.