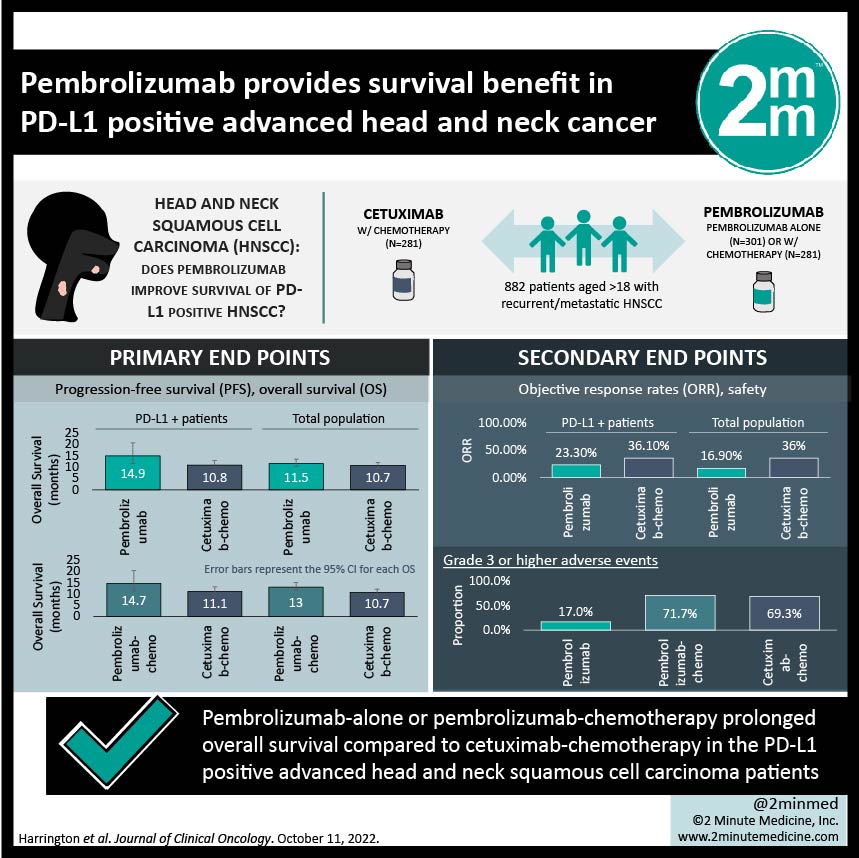
1. Pembrolizumab-alone or pembrolizumab-chemotherapy prolonged overall survival vs cetuximab-chemotherapy in the PD-L1 positive population
2. While pembrolizumab-chemotherapy found an improved response rate compared to cetuximab-chemotherapy, pembrolizumab-alone had a decreased response rate.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Recurrent or metastatic head and neck squamous cell carcinomas (HNSCCs) has previously been treated with platinum-based chemotherapy and cetuximab, but recently have seen success with programmed death 1 inhibitors such as pembrolizumab. First-line pembrolizumab with/without chemotherapy was compared to cetuximab with chemotherapy in a phase III study (KEYNOTE-048), and it was found that pembrolizumab with/without chemotherapy prolonged overall survival (OS) and duration of response (DOR). However, that study only had a follow-up of 1 year. This current study is a post hoc analysis of the previous study but with a 4-year follow-up to determine the long-term impact of pembrolizumab. Primary endpoints included progression-free survival (PFS) and OS. Secondary endpoints included objective response rates (ORR) and safety. This study found that pembrolizumab-alone prolonged OS vs cetuximab-chemotherapy in the PD-L1 positive populations (14.9 months vs 10.8 months) and was non-inferior in the total population (11.5 months vs 10.7 months). Pembrolizumab-chemotherapy prolonged OS vs cetuximab-chemotherapy in both the PD-L1 positive population (14.7 months vs 11.1 months) and the total population (13.0 months vs 10.7 months). With regards to ORR, pembrolizumab-alone did not improve ORR compared with cetuximab-chemotherapy in either the PD-L1 population or total populations, but pembrolizumab-chemotherapy had higher or similar ORR in both populations. Limitations to this study included the nature of this post-hoc analysis. The strengths of this study included its large sample size, its randomization, and its long-term analysis. Overall, the update to KEYNOTE-048 study demonstrates a meaningful survival benefit using immune checkpoint inhibitors in advanced head and neck squamous cell carcinoma even in lieu of chemotherapy confirming that 1st line therapy should strongly consider PD-1/PD-L1 blockade.
Click to read the study in JCO
In-Depth [randomized controlled trial]: This was a phase III trial with 882 patients (aged >18) that had recurrent/metastatic HNSCC and were randomly allocated with stratification based on PD-L1 expression, p16 expression, end ECOG, to pembrolizumab alone (n = 301), pembrolizumab-chemotherapy (n = 281), and cetuximab-chemotherapy (n = 300). Chemotherapy in both groups included cisplatin or carboplatin and fluorouracil. The response was assessed per RECIST by blinded review. The median follow-up time was 45 months. This study found median OS in the pembrolizumab alone group was 14.9 months (95%CI, 11.5 to 20.6) vs 10.8 months (95%CI, 8.8 to 12.8) in the cetuximab-chemotherapy group, and ORR was 23.3% (11CR/20PR) vs 36.1% (4CR/40PR) respectively in PD-L1 positive populations. In the total population, median OS was 11.5 months (95%CI, 10.3 to 13.5) vs 10.7 months (95%CI, 9.3 to 12.1) and ORR was 16.9% (15CR/36PR) vs 36% (8CR/100PR), respectively. The median OS in the pembrolizumab-chemotherapy group was 14.7 months (95%CI, 10.3 to 19.3) vs 11.1 months (95%CI, 9.2 to 13.0) in the cetuximab-chemotherapy group and ORR was 43.7% (13CR/42PR) vs 38.2% (4CR/38PR) respectively in PD-L1 positive populations. In the total population, the median OS was 13.0 months (95%CI, 10.9 to 14.7) vs 10.7 months (95%CI, 9.3 to 11.7) and the ORR was 36.3% (18CR/84PR) vs 36.3% (8CR/93PR) respectively. chemotherapy. With regards to grade 3 or higher adverse events, 17% were reported in the pembrolizumab alone group, 71.7% in the pembrolizumab-chemotherapy, and 69.3% in the cetuximab-chemotherapy group. Overall, long-term follow-up of the KEYNOTE-048 study found favourable results with pembrolizumab with/without
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.