Photo Credit: Myboxpra
The following is a summary of “Diaphragm thickness modifications and associated factors during VA-ECMO for a cardiogenic shock: a cohort study,” published in the March 2024 issue of Critical Care by Moury et al.
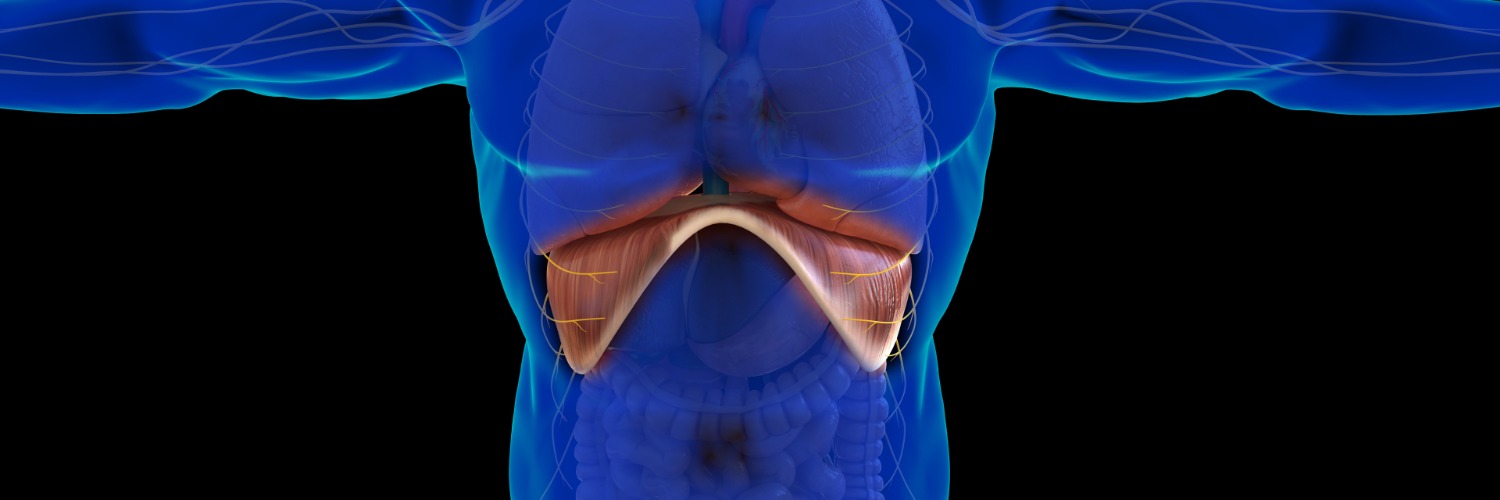
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for cardiogenic shock raises questions about diaphragm thickness changes and their impact.
Researchers conducted a retrospective study investigating how a patient’s diaphragm thickness changes during the first week of VA-ECMO and how it relates to sweep gas flow settings.
They conducted a prospective observational study in a 12-bed ICU in France, enrolling patients undergoing VA-ECMO implantation. The diaphragm thickness and thickening fraction (dTF, where dTF < 20% indicated low contractile activity) were measured daily for one week using ultrasound. Factors affecting diaphragm thickness changes, early extubation (< day 4), and 60-day patient outcomes were studied. Diaphragm thickness changes were analyzed using a mixed-effect linear model (MLM).
The result showed that of 29 patients examined, 23% experienced diaphragm atrophy, 60% remained stable, and 17% showed an increase. None of the 13 early-extubated patients encountered diaphragm atrophy, contrasting with 46% of those extubated later (P-value = 0.008). Diaphragm thickness alterations were unrelated to dTF (P-value = 0.13) but correlated with sweep gas flow (Beta = − 3; 95% CI [− 4.8; − 1.2], P-value = 0.001) and pH (Beta = − 2; 95% CI [− 2.9; − 1], P-value < 0.001) in MLM. By the study’s conclusion, 69% of patients maintained a low dTF (< 20%), which was associated with sweep gas flow evolution in MLM (Beta = − 2.8; 95% CI [− 5.2; − 0.5], P-value = 0.017). The OR of death within 60 days for those exhibiting diaphragm atrophy by day 7 was 8.50 (95% CI [1.4–74], P=0.029).
They concluded that frequent changes in diaphragm thickness during VA-ECMO, with early extubation and adjusted sweep gas flow, linked to better muscle preservation in survivors.
Source: annalsofintensivecare.springeropen.com/articles/10.1186/s13613-024-01264-8